Abstract
Background
Our aim was to describe the relationship between risk factors, such as stress, depression, and anxiety and potentially protective factors against pediatric headache-related disability, such as mindfulness, resilience, and self-compassion, and to determine teens’ interest in mind-body skills training to help reduce headache-related disability.
Methods
This was a cross-sectional survey among adolescents seen in an academic neurology clinic reporting four or more headaches monthly using standardized instruments to determine the relationship between putative risk and protective factors as well as physiologic markers of inflammation and vagal tone and headache-related disability.
Results
Among the 29 participants, 31% were male, the average age was 14.8 years, average headache frequency was 11.6 per month, and the most commonly reported trigger was stress (86%). The only risk or protective factor significantly associated with headache-related disability was depression (r=0.52, P=0.004). Depression was negatively correlated with mindfulness, resilience, and self-compassion (P<0.01 each) and positively correlated with stress, sleep disturbance, and anxiety (P<0.01 each). Biomarkers of vagal tone and inflammation were correlated with each other, but not with headache-related disability or depression. There was strong interest in learning skills like slow, deep breathing practices supported by a smart phone app to reduce stress and the negative impact of headaches on daily life.
Discussion
Among teens with frequent migraine headaches, depression is the strongest risk factor for headache-related disability. Stress is viewed as a headache trigger, and teens reported wanting to learn simple stress management strategies supported by a smart phone application to help reduce headache-related disability.
Keywords: headache, migraine, pediatric, mind-body, stress, depression, anxiety, disability, heart rate variability, inflammation
Background
Headaches are a common pediatric problem with serious impact on overall health status, quality of life, and disability.1, 2 Tension-type headache (TTH) is the most common with prevalence rates ranging from 10 to 25 percent in school-aged children.3 Migraine headaches are somewhat less common with prevalence ranging from about 3 percent in younger school-aged children to nearly 20 percent in older adolescents.3
Risk factors thought to increase the adverse impact of headaches in both adults and teens include anxiety, depression, stress, and sleep disturbances; 4, 5, 6 addressing these risk factors is an important treatment goal in overall headache management. Protective factors that may lessen the impact of headache-related disability have not been as well characterized in pediatric populations. Adult studies suggest that mindfulness (defined as moment-to-moment nonjudgmental awareness)7 and self-compassion (defined as kindness toward self, mindfulness, and a sense of common humanity with others) 8 are associated with decreased stress, anxiety, depression, perceptions of pain, and disability due to pain. 8–11 Furthermore, training in mindfulness and self-compassion can improve these factors for patients with chronic pain; in addition, training in these mind-body techniques affects brain physiology and function, autonomic function, telomerase activity, and inflammatory biomarkers. 12–16
Although studies in adolescents suggest that mind-body skills’ training is possible, 17 the effects of training in mindfulness and self-compassion have not been tested for adolescents with recurrent migraine headaches. Other mind-body techniques, such as self-hypnosis and therapist-administered biofeedback, have proven useful in preventing headaches in youth, 18–21 but have not been universally adopted. In our tertiary care setting, some of these techniques are offered, but additional techniques to reduce stress and improve overall resilience and mental health may be useful and desirable.
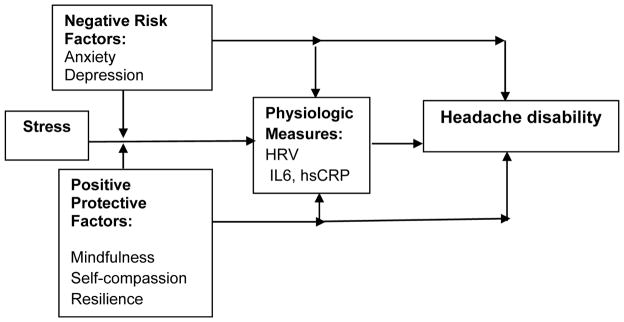
Before embarking on a study to determine the benefits of teaching mind-body skills to adolescents with chronic recurrent headaches, we wished to test the conceptual model underlying this intervention (Figure 1) and to more specifically assess teens’ interest in mind-body training.
Figure 1.
Preliminary Conceptual Model Showing Expected Relationships Among Risk, Protective, and Physiologic Variables Affecting the Impact of Headaches on Functional Status in Adolescents
Specifically, we wished to characterize the relationships between: potentially a) negative risk factors (stress, anxiety, and depression); b) positive protective factors (mindfulness, self-compassion); and c) physiologic markers of inflammation and autonomic balance (heart rate variability, HRV; interleukin-6, and high sensitivity C-reactive protein) on d) headache impact. If positive protective factors were found to be associated with lower impact of headache, subsequent studies could focus on enhancing these qualities. If not, future interventions might better focus on other factors. Finally, we wished to describe teens’ interest in learning additional mind-body skills, and to determine which factors were most important to them in considering additional therapies.
Methods
This was a cross-sectional study conducted at a large Midwestern pediatric teaching hospital between May and November, 2014. Participants were recruited through flyers in the hospital’s Neurology Clinic, Pain Clinic, and GI Clinic. In addition, the electronic medical records were searched, and patients whose records indicated a diagnosis of migraine headache were sent a letter inviting them to participate. Subjects were offered a gift of $25 for completing one study visit which included answering multiple questionnaires, having their blood drawn, and having their heart rate monitored for about 20 minutes. Our goal was to recruit 30 participants over 6 months.
Subjects were eligible if they were 12–18 years old and reported a primary diagnosis of migraines with or without aura, chronic migraines, or chronic TTH with a frequency of at least 4 headaches per month. Potential participants were excluded if they were currently enrolled in other headache studies at the institution, received onabotulinumtoxin A within 3 months for headache management; had secondary headaches associated with head trauma, brain tumors, or any other diagnoses that in the opinion of the study neurologists might make them unsuitable candidates for the study or for whom the study itself would interfere with their medical care.
Study visits were scheduled separately from clinic visits and were held in the Children’s Hospital’s Clinical Research Center between 9 and 11am to minimize diurnal variability. After obtaining informed consent from parents and assent from participants, participants completed study questionnaires, had blood drawn, and underwent continuous cardiac monitoring for 20 minutes while at rest to obtain data for heart rate variability (HRV) analysis. Questionnaire data were completed using REDCap, cleaned by the study coordinator, and exported to Microsoft Excel for transmission to the project biostatistician for analysis. Blood samples were flash frozen and sent to the lab for batch analysis.
Standard questionnaires were used to measure the main study outcome (headache disability) and proposed negative and positive predictive factors.
Headache disability was assessed using the Headache Impact Test (HIT)-6 (which is validated assessment of the functional impact of headaches for both migraine and TTH in adults and has been used in adolescent as well as adult headache populations). 22–24 It has high reliability and correlates well with the Migraine Disability Assessment Scale, headache pain severity, and the number of headaches per month.23, 25–27 It is also sensitive enough to detect improvements associated with pharmacologic and non-pharmacologic treatments. 28–30 Scores over 55 indicate severe headache-related disability. 31
Negative (risk) factors
Stress was assessed with the widely used Cohen’s 10-item Perceived Stress Scale.32 Anxiety and depression were assessed using the NIH-funded Patient Reported Outcome Measurement Information System (PROMIS) Short-Form Pediatric Anxiety Scale and the PROMIS Short-Form Pediatric Depression Scale.33, 34 Sleep disturbance was measured using the PROMIS Sleep Disturbance Scale, in which higher scores indicate greater sleep disturbance. 35
Positive (protective) factors
Mindfulness was assessed using the 10-item version of the Cognitive and Affective Mindfulness Scale, Revised,36 and self-compassion was assessed using the 12-item version of Neff’s Self-Compassion Scale.37 Resilience was assessed using Smith’s 6–item Brief Resilience Scale.38
Intermediate Physiologic factors
Autonomic balance was assessed with two measures of heart rate variability (HRV), the standard deviation of the interbeat interval (SDNN) and the root mean square of the successive differences (RMSSD) which is thought to represent primarily vagal tone. HRV was assessed with a 20 minute recording in the Clinical Research Center, with the subject lying supine with the head of the bed raised 45 degrees. ECG and impedance measures were obtained using a Bionex system (Mindware, Gahanna, OH). The electrocardiogram was performed in the standard lead II configuration. Following technician analysis for artifact and ectopy, the ECG results were downloaded into a computer software program that produces frequency domain variables [Total power spectrum (0–0.4 Hz), high frequency (HF) power (0.15–0.4 Hz), and low frequency (LF) power (0.04–0.15 Hz)]. The middle ten minutes of the recordings were scored minute by minute and then averaged over the ten minute period. HRV data were exported into an EXCEL database and sent to the study biostatistician for analysis.
Blood specimens were collected by a research nurse and stored in the Clinical Research Center freezers at −80 degrees Fahrenheit before being sent in batches to the Nationwide Children’s Hospital laboratory on dry ice. Inflammatory biomarkers, high sensitivity C-reactive protein (hsCRP) and interleukin 6 (IL-6) were measured using the ELISA based platform by Meso Scale Discovery. Samples were applied directly into the wells of a 96 well plate (IL-6, cat # K151QXD and hsCRP, cat # K151STD) and read on an MSD Sector S600 Instrument. Sample concentrations were calculated using an experimentally derived standard curve and expressed as pg/mL serum.
Data analysis
Due to the small sample size in this pilot study, structural equation modeling, factor analysis and principal component analysis could not be used to test the overall model. Instead, we performed descriptive statistics on standard measures of perceived stress, anxiety, depression, mindfulness, self-compassion, heart rate variability, hsCRP and IL6. We also calculated the Pearson’s correlation coefficient or Spearman (depending on data distribution) correlation coefficient between headache disability and each standard outcome measure.
This study was approved by the Nationwide Children’s Hospital Institutional Review Board.
Results
Between May 1 and November 30, 2014, 29 participants were recruited. Their demographic and headache characteristics are summarized with simple descriptive statistics in Table 1. They had an average age of 15 years with an average of a 5 year history of headaches, and most were female. Nearly all (97%) had migraine headaches and a substantial percentage (45%) also had tension-type headache. Typical headache triggers included stress, fatigue, and dehydration. Average headache severity in the week prior to survey completion was 6.2 on a 0 to 10 Visual Analog Scale (0 = none). On average, participants had experienced recurrent headaches for over 5 years, and reported nearly a dozen headaches per month. The average 6-item Headache Impact Test (HIT-6) score was 64, indicating severe functional impact of headaches.25
Table 1.
Participant Demographic and Headache Characteristics
| Characteristic/Instrument | Values (Mean ± SD, N (%)) |
|---|---|
| Demographic characteristics | |
| Age in Years | 14.8 ± 2 years |
| Gender (% male) | 31% |
| Race (% Caucasian) | 76% |
| % African American | 17% |
| Hours of sleep per might | 8.4 ± 1.4 |
| TV/Screen time : TV Time daily | 2.5 ± 1.9 hours |
| Computer time daily | 3.6 ± 2.5 hours daily |
| Headache characteristics | |
| Headache Impact Test (HIT-6) SCORE | 64 ± 6.2 |
| Type of Headache (N, % each) | |
| Migraines | 97% |
| Tension-Type HA | 45% |
| Other (tumor, dental, genetic, vision, Injury, Other) | 17% |
| More than 1 type of HA | 45% |
| Headache Severity in Past week (0–10) | 6.2 ± 2.4 |
| Years of chronic HA | 5.5 ± 3.4 |
| # HA per month | 11.6 ± 8.5 |
| School days missed due to HA in past 30 days | 2.4 ± 4.9 |
| HA Triggers Identified by Teens | |
| Stress | 86% |
| Fatigue | 55% |
| Weather changes | 48% |
| Dehydration | 48% |
Table 2 shows participants’ other health characteristics. On the PROMIS Anxiety Scale, the average T-score was 60.3 and the PROMIS Depression T-score was 48.2 (population norm is 50 on both scales). The most commonly cited health goals were less pain (66%), better sleep (66%), and better concentration (62%); the most common health conditions other than headache were sleep problems, back pain, and depressed mood. Most (76%) participants used one or more prescription medications, and 59% reported using one or more dietary supplements, most often multivitamins (33%), magnesium (28%) or riboflavin (10%); none reported using feverfew (Tanacetum parthenium), butterbur (Petasites hybridus), or coenzyme Q10.
Table 2.
Other Health Characteristics and Health Care
| Other Health | |
|---|---|
| Number of other health problems | 3.6 ± 2.5 |
| Sleep | 41% |
| Back pain | 35% |
| Mood | 35% |
| Health Goals | |
| Less pain | 66% |
| Better sleep | 66% |
| Better concentration | 62% |
| PROMIS Scales (T-score, 50 ± 10 is population mean; higher scores are worse) | |
| Anxiety | 60.3 ± 9.3 |
| Depression | 48.2 ± 11.4 |
| Sleep Disturbance | 52.2 ± 5 |
| Medications and Supplements | |
| Medications (% using any) | 76% |
| Dietary Supplements (% any) | 59% used 1 or more |
| Multivitamins | 33% |
| B vitamins (B2, riboflavin) | 10% |
| Magnesium | 28% |
| Fish oil | 3% |
| Butterbur, Coenzyme Q10, Feverfew | 0 |
Participants reported moderately high levels of stress with a Perceived Stress Score of 19.6 ± 7.6 (population normative values range from 12–14) and an average stress level of 6.0 on a 0 – 10 scale (0 = none) (Table 3). Recent stressful events affected two thirds of participants, most commonly bullying (48%), having a household member with a chronic illness or disability (35%), or living in more than one home (28%). Most reported that stress triggered or worsened headaches. Relaxation strategies used by 50% or more of the participants included going to bed (83%), listening to music (69%), and/or watching TV (55%). Only 17% reported using a stress management application (app) on a smart device (phone or tablet).
Table 3.
Stress, Relaxation Strategies, Protective Factors, and Physiologic Biomarkers
| Stress | |
|---|---|
| Perceived Stress Scale (10-item score) | 19.6 ± 7.6 |
| Stress VAS (0–10) | 6.0 ± 2.1 |
| Recent stressful event (bullying (48%); household member with chronic illness or disability (35%), multiple homes (28%)) | 66% |
| Stress triggers HA (% yes) | 79% |
| Stress worsens HA (%yes) | 79% |
| Relaxation strategies | |
| Go to bed | 83% |
| Listen to music | 69% |
| Watch TV | 55% |
| Protective factors | |
| Mindfulness (CAMS-R) – item mean | 2.48 ± 0.6 |
| Self-Compassion Scale (Score of 12 items) | 38.9 ± 7.5 |
| Smith’s Brief Resilience Scale (score of 6 items) | 19.6 ±3.8 |
| Physiologic Measures | |
| Biomarkers | |
| IL-6 (in picograms/mL) | 0.31 ± 0.27 |
| hsCRP (in picograms/mL) | 2595.6 ± 6141.4 |
| HRV | |
| HR (mean) | 70.5 ± 11.5 |
| RSA (mean) | 7.33 ± 1.6 |
| SDNN | 83.5 ± 42 |
| RMSSD | 96 ± 64.7 |
| LF | 993.5 ± 722.8 |
| HF/RSA power | 3381.6 ± 3429.9 |
Average scores on the factors thought to be protective (mindfulness, self-compassion, and resilience) and physiologic biomarkers are displayed in Table 3. Mindfulness scores were similar to those reported in depressed adults;39 average self-compassion scores were lower than those in the general population;37 and mean resilience scores were lower than those in the general population.38
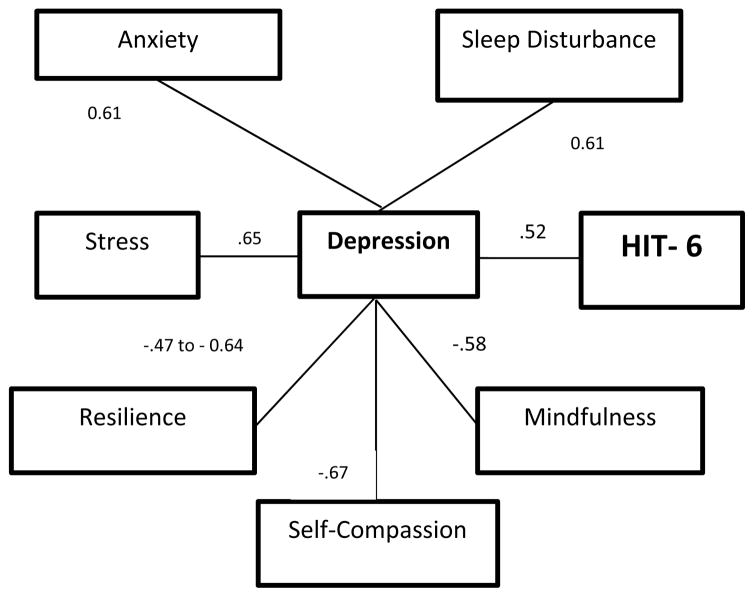
Correlations among factors are shown in Tables 4 and 5, and key correlations between hypothesized factors are displayed in Figure 2. The only factor significantly correlated with the HIT-6 score was depression (r= 0.52, P=0.0006). Depression was positively correlated with stress, anxiety, and sleep disturbance (P<0.01 for each), and negatively correlated with mindfulness, self-compassion, and resilience (P<0.01 for each) (Table 4).
Table 4.
Correlations Between Headache Impact Test (HIT-6) and Other Factors
| HIT6 | Depression | Stress | Anxiety | Sleep Disturb | Mindfulness | Self-Compassion | Resilience | |
|---|---|---|---|---|---|---|---|---|
| HIT6 | 1.0000 | 0.5184 | 0.1716 | 0.3214 | 0.1323 | −0.3295 | −0.1852 | −0.1748 |
| p-value | 0.0040 | 0.3735 | 0.0891 | 0.5020 | 0.0869 | 0.3363 | 0.3644 | |
| Depression | 0.5184 | 1.0000 | 0.6494 | 0.6066 | 0.6078 | −0.5786 | −0.6725 | −0.6374 |
| p-value | 0.0040 | 0.0001 | 0.0005 | 0.0006 | 0.0013 | <.0001 | 0.0002 | |
| Stress | 0.1716 | 0.6494 | 1.0000 | 0.4946 | 0.5103 | −0.6130 | −0.7105 | −0.7518 |
| p-value | 0.3735 | 0.0001 | 0.0064 | 0.0055 | 0.0005 | <.0001 | <.0001 | |
| Anxiety | 0.3214 | 0.6066 | 0.4946 | 1.0000 | 0.5391 | −0.1966 | −0.4142 | −0.6391 |
| p-value | 0.0891 | 0.0005 | 0.0064 | 0.0031 | 0.3161 | 0.0255 | 0.0002 | |
| Sleep Disturbance | 0.1323 | 0.6078 | 0.5103 | 0.5391 | 1.0000 | −0.3068 | −0.4303 | −0.4149 |
| p-value | 0.5020 | 0.0006 | 0.0055 | 0.0031 | 0.1196 | 0.0223 | 0.0282 | |
| Mindfulness | −0.3295 | −0.5786 | −0.6130 | −0.1966 | −0.3068 | 1.0000 | 0.3831 | 0.4039 |
| p-value | 0.0869 | 0.0013 | 0.0005 | 0.3161 | 0.1196 | 0.0442 | 0.0331 | |
| Self-Compassion Scale | −0.1852 | −0.6725 | −0.7105 | −0.4142 | −0.4303 | 0.3831 | 1.0000 | 0.5564 |
| p-value | 0.3363 | <.0001 | <.0001 | 0.0255 | 0.0223 | 0.0442 | 0.0017 | |
| Resilience | −0.1748 | −0.6374 | −0.7518 | −0.6391 | −0.4149 | 0.4039 | 0.5564 | 1.0000 |
| p-value | 0.3644 | 0.0002 | <.0001 | 0.0002 | 0.0282 | 0.0331 | 0.0017 |
HIT6 refers to the 6-item Headache Impact Test; Depression, Anxiety, and Sleep Disturbance were measured using the PROMIS instruments; Stress was measured with the 10-item Perceived Stress Scale; Mindfulness was measured using the 10-item Cognitive and Affective Mindfulness Scale – Revised; Self-Compassion was measured using the 12-item Short Self-Compassion Scale; Resilience was measured using Smith’s 6-item Resilience Scale. P-values less than 0.05 are noted in bold font.
Table 5.
Pearson Correlations Among Biomarkers and Psychological Factors
| SDNN | RMSSD | IL6 | HSCRP | |
|---|---|---|---|---|
| HIT6 | −0.2582 | −0.1576 | −0.0021 | −0.2405 |
| p-value | 0.1763 | 0.4142 | 0.9914 | 0.2088 |
| Depression | −0.1937 | −0.2378 | −0.0289 | −0.1512 |
| p-value | 0.3141 | 0.2142 | 0.8815 | 0.4337 |
| Stress | 0.0787 | 0.0949 | 0.1665 | 0.1431 |
| p-value | 0.6850 | 0.6243 | 0.3879 | 0.4590 |
| Anxiety | −0.1663 | −0.1741 | 0.0129 | 0.0201 |
| p-value | 0.3886 | 0.3664 | 0.9471 | 0.9176 |
| Sleep Disturbance | −0.2592 | −0.2806 | 0.2121 | 0.3655 |
| p-value | 0.1828 | 0.1480 | 0.2787 | 0.0558 |
| Mindfulness | −0.0021 | 0.0157 | −0.2363 | −0.0319 |
| p-value | 0.9914 | 0.9367 | 0.2260 | 0.8721 |
| Self-Compassion | −0.2158 | −0.1701 | 0.0516 | 0.0559 |
| p-value | 0.2610 | 0.3777 | 0.7903 | 0.7734 |
| Resilience | 0.0803 | 0.1057 | 0.0824 | −0.0515 |
| p-value | 0.6787 | 0.5853 | 0.6710 | 0.7910 |
| SDNN | 1.0000 | 0.9583 | 0.1175 | 0.2628 |
| p-value | <.0001 | 0.5440 | 0.1684 | |
| RMSSD | 0.9583 | 1.0000 | 0.1372 | 0.2620 |
| p-value | <.0001 | 0.4778 | 0.1698 | |
| IL6 | 0.1175 | 0.1372 | 1.0000 | 0.7876 |
| p-value | 0.5440 | 0.4778 | <.0001 | |
| HSCRP | 0.2628 | 0.2620 | 0.7876 | 1.0000 |
| p-value | 0.1684 | 0.1698 | <.0001 |
SDNN refers to the standard deviation of the interbeat interval (heart rate variability); RMSSD refers to root mean square of successive differences which is a vagally-mediated measure of heart rate variability; IL-6 (interleukin-6) and HSCRP (high sensitivity C-reactive protein) are inflammatory biomarkers
Figure 2. Relationships and Correlations in Relationship to HIT-6.
The only factor significantly directly associated with HIT-6 score is PROMIS Depression score.
Factors significantly related to increased risk of depression include worse sleep (r=0.61), perceived stress (r=0.64), and anxiety (r=0.61).
Factors significantly related to decreased risk of depression include mindfulness (r=.58), self-compassion (r=0.67), and resilience (r=0.47 to 0.64 for OSU and Brief Resilience Scales respectively). In addition, mindfulness is related to lower stress; and self-compassion is related to lower stress, anxiety, and poor sleep.
None of the biomarkers (HRV, IL-6, or hsCRP) were significantly related to HIT-6 or Depression
All noted correlations are significant at P<0.01
Inflammatory biomarkers and vagal tone were not associated with HIT-6 or depression scores (Table 5).
In answer to the questions about future intervention studies, 86% of respondents expressed interest in learning additional stress management skills. The preferred skill was slow, deep breathing (72%); only 21% wanted to learn the Relaxation Response or use biofeedback, and none were interested in mindfulness meditation or Transcendental Meditation. The most important factors in stress management training were convenience, existing scientific evidence of effectiveness, and being able to get to know a teacher personally. Most participants (60%) were willing to spend 2 hours learning a new mind-body skill, and over 70% were willing to practice 20 minutes daily if it would help decrease headache frequency or severity. The favorite strategy for home practice was a smart phone app (favored by 72% of participants), followed by YouTube videos (38%) and MP3 recordings (29%).
Discussion
Our patients were typical of adolescents seen in Pediatric Neurology Clinics for migraine treatment. They had multi-year histories of recurrent headaches, with an average pain severity of 6 on a 10 point scale in the past week and severe functional impairment from headaches. Although they reported a variety of health problems, health goals, and stressors, the only measured risk or protective factor significantly related to the functional impact of headaches on their lives was depression. While neither inflammatory nor autonomic biomarkers were significantly related to either headache impact or depression, multiple other factors were related to depression. Mindfulness, self-compassion, and resilience were negatively associated with depression, while anxiety, stress and sleep disturbances were positively associated with depression. Finally, although they identified a number of stress-management strategies they already used, these teens wanted additional training and they had very specific ideas of what kind of mind-body training they wanted – training that required less than 2 hours, focusing on slow, deep breathing, and training that would be supported by a smart phone app.
Our results are consistent with those of other studies evaluating risk factors such as depression for migraine and TTH in children. For example, in a prospective cohort study in New Zealand, risk factors for migraine and TTH in 11 year old children included sleep duration, maternal smoking, and having been bullied. 40 Depression is a well-known independent predictor of moderate to severe headache-related disability among adolescents.41 High levels of impairment from pain due to pediatric migraine are associated with increased depression, older age, multiple pain sources, and prior hospitalization for pain. In another study, adolescents who missed more school due to headache had higher depression scores and lower academic performance than students who missed less school.42 When tricyclic antidepressants are used to treat migraine and other types of chronic pain, they are typically in lower doses than when used primarily for depression. 43 It would be worthwhile to conduct additional research to determine the impact of explicit treatment of depressive symptoms on headache-related disability in adolescents.
As in other studies, our participants reported a high rate of sleep disorders.44 A population study of headache and sleep problems in adults showed concurrence in 18% of the population, most often with high stress, and among women and participants with low SES and poor quality of life.45 Fatigue may be a trigger for migraines and for inducing hyperalgesia. 46, 47 This suggests that lifestyle and mind-body interventions targeting sleep may be a fruitful area for research on mitigating the effects of migraine on quality of life.
As in previous studies, participants in our sample reported a high rate of using complementary therapies. Previous research suggests that among headache patients, use of mind-body therapies is second only to dietary supplements.48 We were not surprised that participants reported using magnesium and riboflavin since these are adjunctive treatments recommended by many neurologists and other physicians for migraine headaches in adults; 49, 50 however, it was surprising that none of the adolescents in our sample reported using other commonly used herbs and supplements for headache such as butterbur, feverfew and Coenzyme Q10.
Although the correlation analysis did not show that stress was strongly correlated to headache impact, our participants identified stress as both a headache trigger and a factor that made headaches worse, and they wanted additional mind-body training to help manage stress. Several studies have suggested that yoga is a promising stress management strategy to prevent migraines. For example, one study offered yoga 5 days per week for 6 weeks plus conventional care compared with conventional care alone; the yoga showed better improvement in clinical outcomes and better HRV.51 Another study showed significant improvement with yoga in pediatric patients with chronic pain.52 In adults, mindfulness training has shown promise for helping lower stress and migraine frequency.53 Other studies have shown that biofeedback and relaxation therapy can be helpful for migraine patients, especially when co-morbidities such as depression or anxiety are present;54 however these interventions are time intensive and necessitate the involvement of trained providers to instruct patients on these modalities. Given choices about different types of mind-body training, our participants expressed the strongest interest in simple slow, deep breathing exercises supported by a smart phone app. Little research has explored the potential for this intervention in pediatric patients.
Limitations
This study had several limitations. First, it had a modest sample and drew from one tertiary care institution in the Midwest. It did not include children 12 years and younger. It focused on patients with a history of migraine, many of whom had multiple kinds of headache, but its findings cannot be extrapolated to the large population of children suffering from episodic or frequent tension type headache or post-traumatic headaches. As a cross-sectional study, it cannot confirm causal relationships. The survey inquired about interest in mind-body skills training but did not ask participants if they would like to try other non-pharmacologic therapies such as dietary supplements, osteopathic manipulative therapy, or acupuncture. It also did not ask adolescents if they would like referral to mental health specialists or social workers to address issues related to depression, anxiety, or stressful living conditions. Larger, multi-center studies are needed to address these questions.
Conclusion
Despite these limitations, this study offers important insights for clinical care and future studies. Among all the risk and protective factors we evaluated, depression was most strongly linked to poor functional status related to headache. Teens recognized stress as a trigger for their headaches and reported wanting more training in mind-body strategies like slow, deep breathing supported by smart phone apps to help manage their headaches. Future studies can evaluate whether their desired approach indeed improves headache-related disability and overall quality of life.
Acknowledgments
This project was supported in part by Award Number Grant UL1TR001070 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health. We are grateful to Grace Wentzel and Paulla Dennis at the Nationwide Children’s Hospital Clinical Research Services office and the Research Institute at NCH for assistance with project management.
We are grateful to Julianne Arthur Shelli Farley, and Katlyn Latimer, for their assistance in identifying, recruiting, enrolling patients and collecting data. We are grateful to Dr. Lynette Rogers for analysis of the blood samples for high sensitivity C-reactive protein and Interleukin-6. We are grateful to Melissa Moore-Clingenpeel and Igor Dvorchik from the Nationwide Children’s Hospital Biostatistics Core for assistance with data analysis.
Funding: This project was funded by an intramural grant from the Research Institute at Nationwide Children’s Hospital. In-kind faculty support was provided by the Ohio State University College of Medicine.
Footnotes
Conflicts of interest: none
Authorship: All authors made substantial contributions to the conception and design of this study and approve the final manuscript. PB lent the equipment and contributed to the design and analysis of the data related to heart rate variability and edited and approved the final version of the manuscript. GH and AP contributed to design, participant recruitment, writing and editing the manuscript and final approval of the manuscript. KK designed the study, led the research team in obtaining intramural funding, IRB approval, supervising the study coordinators, interfacing with laboratory and statistical analytic staff, writing, editing, and submitting the manuscript.
References
- 1.Bethell C, Kemper KJ, Gombojav N, Koch TK. Complementary and conventional medicine use among youth with recurrent headaches. Pediatrics. 2013;132:e1173–83. doi: 10.1542/peds.2013-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferracini GN, Dach F, Speciali JG. Quality of life and health-related disability in children with migraine. Headache. 2014;54:325–34. doi: 10.1111/head.12251. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52:1088–97. doi: 10.1111/j.1469-8749.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- 4.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurologic clinics. 2009;27:321–34. doi: 10.1016/j.ncl.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Arruda MA, Bigal ME. Migraine and migraine subtypes in preadolescent children: association with school performance. Neurology. 2012;79:1881–8. doi: 10.1212/WNL.0b013e318271f812. [DOI] [PubMed] [Google Scholar]
- 6.Pakalnis A, Splaingard M, Splaingard D, Kring D, Colvin A. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49:1486–92. doi: 10.1111/j.1526-4610.2009.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. Jama. 2008;300:1350–2. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- 8.Neff KD. The Development and Validation of a Scale to Measure Self-Compassion. Self and Identity. 2003;2:223–50. [Google Scholar]
- 9.Rasmussen MK, Pidgeon AM. The direct and indirect benefits of dispositional mindfulness on self-esteem and social anxiety. Anxiety, stress, and coping. 2011;24:227–33. doi: 10.1080/10615806.2010.515681. [DOI] [PubMed] [Google Scholar]
- 10.Wren AA, Somers TJ, Wright MA, et al. Self-Compassion in Patients With Persistent Musculoskeletal Pain: Relationship of Self-Compassion to Adjustment to Persistent Pain. J Pain Symptom Manage. 2011 doi: 10.1016/j.jpainsymman.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Wren AA, Somers TJ, Wright MA, et al. Self-compassion in patients with persistent musculoskeletal pain: relationship of self-compassion to adjustment to persistent pain. J Pain Symptom Manage. 2012;43:759–70. doi: 10.1016/j.jpainsymman.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–49. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Gard T, Holzel BK, Sack AT, et al. Pain Attenuation through Mindfulness is Associated with Decreased Cognitive Control and Increased Sensory Processing in the Brain. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner-Nix J, Backman S, Barbati J, Grummitt J. Evaluating distance education of a mindfulness-based meditation programme for chronic pain management. J Telemed Telecare. 2008;14:88–92. doi: 10.1258/jtt.2007.070811. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–81. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Sibinga EM, Kerrigan D, Stewart M, Johnson K, Magyari T, Ellen JM. Mindfulness-based stress reduction for urban youth. J Altern Complement Med. 2011;17:213–8. doi: 10.1089/acm.2009.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen KD. Using biofeedback to make childhood headaches less of a pain. Pediatr Ann. 2004;33:241–5. doi: 10.3928/0090-4481-20040401-10. [DOI] [PubMed] [Google Scholar]
- 19.Kang EH, Park JE, Chung CS, Yu BH. Effect of biofeedback-assisted autogenic training on headache activity and mood states in Korean female migraine patients. J Korean Med Sci. 2009;24:936–40. doi: 10.3346/jkms.2009.24.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohen DP. Chronic daily headache: helping adolescents help themselves with self-hypnosis. Am J Clin Hypn. 2011;54:32–46. doi: 10.1080/00029157.2011.566767. [DOI] [PubMed] [Google Scholar]
- 21.Rains JC. Change mechanisms in EMG biofeedback training: cognitive changes underlying improvements in tension headache. Headache. 2008;48:735–6. doi: 10.1111/j.1526-4610.2008.01119_1.x. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 22.Rendas-Baum R, Yang M, Varon SF, Bloudek LM, DeGryse RE, Kosinski M. Validation of the Headache Impact Test (HIT-6) in patients with chronic migraine. Health Qual Life Outcomes. 2014;12:117. doi: 10.1186/s12955-014-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6) across episodic and chronic migraine. Cephalalgia. 2011;31:357–67. doi: 10.1177/0333102410379890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton RB, Manack A, Ricci JA, Chee E, Turkel CC, Winner P. Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in adolescents study (C-dAS) Headache. 2011;51:693–706. doi: 10.1111/j.1526-4610.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawata AK, Coeytaux RR, Devellis RF, Finkel AG, Mann JD, Kahn K. Psychometric properties of the HIT-6 among patients in a headache-specialty practice. Headache. 2005;45:638–43. doi: 10.1111/j.1526-4610.2005.05130.x. [DOI] [PubMed] [Google Scholar]
- 26.Piebes SK, Snyder AR, Bay RC, Valovich McLeod TC. Measurement properties of headache-specific outcomes scales in adolescent athletes. Journal of sport rehabilitation. 2011;20:129–42. doi: 10.1123/jsr.20.1.129. [DOI] [PubMed] [Google Scholar]
- 27.Coeytaux RR, Linville JC. Chronic daily headache in a primary care population: prevalence and headache impact test scores. Headache. 2007;47:7–12. doi: 10.1111/j.1526-4610.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 28.Coeytaux RR, Kaufman JS, Chao R, Mann JD, Devellis RF. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol. 2006;59:374–80. doi: 10.1016/j.jclinepi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Castien RF, Blankenstein AH, Windt DA, Dekker J. Minimal clinically important change on the Headache Impact Test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia. 2012;32:710–4. doi: 10.1177/0333102412449933. [DOI] [PubMed] [Google Scholar]
- 30.Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-Armed randomized controlled trial. Complement Ther Med. 2015;23:149–56. doi: 10.1016/j.ctim.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Bayliss M, Batenhorst A. The HIT-6TM: A User’s Guide. Lincoln, RI: QualityMetric, Inc; 2002. [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 33.Irwin DE, Gross HE, Stucky BD, et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes. 2012;10:22. doi: 10.1186/1477-7525-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin DE, Stucky B, Langer MM, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19:595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman G, Hayes A, Kumar S, Greeson J, Laurenceau JP. Mindfulness and emotion regulation: The development and initial validation of the Cognitive and Affective Mindfulness Scale-Revised (CAMS-R) Journal of Psychopathology and Behavioral Assessment. 2007;29:177–90. [Google Scholar]
- 37.Raes F, Pommier E, Neff KD, Van Gucht D. Construction and Factorial Validation of a Short Form of the Self-Compassion Scale. Clinical Psychology & Psychotherapy. 2011;18:250–5. doi: 10.1002/cpp.702. [DOI] [PubMed] [Google Scholar]
- 38.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15:194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Feldman G, Hayes A. Changes in Mindfulness and Emotion Regulation in an Exposure-Based Cognitive Therapy for Depression. Cognitive Therapy and Research. 2008;32:734–44. [Google Scholar]
- 40.Waldie KE, Thompson JM, Mia Y, Murphy R, Wall C, Mitchell EA. Risk factors for migraine and tension-type headache in 11 year old children. The journal of headache and pain. 2014;15:60. doi: 10.1186/1129-2377-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuh JL, Wang SJ, Lu SR, Liao YC, Chen SP, Yang CY. Headache disability among adolescents: a student population-based study. Headache. 2010;50:210–8. doi: 10.1111/j.1526-4610.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- 42.Breuner CC, Smith MS, Womack WM. Factors related to school absenteeism in adolescents with recurrent headache. Headache. 2004;44:217–22. doi: 10.1111/j.1526-4610.2004.04050.x. [DOI] [PubMed] [Google Scholar]
- 43.Hershey AD, Powers SW, Coffey CS, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. 2013;53:799–816. doi: 10.1111/head.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams MA, Aurora SK, Frederick IO, Qiu C, Gelaye B, Cripe SM. Sleep duration, vital exhaustion and perceived stress among pregnant migraineurs and non-migraineurs. BMC pregnancy and childbirth. 2010;10:72. doi: 10.1186/1471-2393-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund N, Westergaard ML, Barloese M, Glumer C, Jensen RH. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia. 2014;34:833–45. doi: 10.1177/0333102414543332. [DOI] [PubMed] [Google Scholar]
- 46.Engstrom M, Hagen K, Bjork MH, et al. Sleep quality, arousal and pain thresholds in migraineurs: a blinded controlled polysomnographic study. The journal of headache and pain. 2013;14:12. doi: 10.1186/1129-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engstrom M, Hagen K, Bjork M, Stovner LJ, Stjern M, Sand T. Sleep quality, arousal and pain thresholds in tension-type headache: a blinded controlled polysomnographic study. Cephalalgia. 2014;34:455–63. doi: 10.1177/0333102413515339. [DOI] [PubMed] [Google Scholar]
- 48.Bethell C, Kemper K, Gombojav N, Koch T. Complementary and conventional medicine use among youth with recurrent headaches. J Altern Complement Med. 2014;20:A30–1. doi: 10.1542/peds.2013-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F, Van Den Eeden SK, Ackerson LM, Salk SE, Reince RH, Elin RJ. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601–10. doi: 10.1046/j.1526-4610.2003.03102.x. [DOI] [PubMed] [Google Scholar]
- 50.Sherwood M, Goldman RD. Effectiveness of riboflavin in pediatric migraine prevention. Can Fam Physician. 2014;60:244–6. [PMC free article] [PubMed] [Google Scholar]
- 51.Kisan R, Sujan M, Adoor M, et al. Effect of Yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126–32. doi: 10.4103/0973-6131.133891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans S, Moieni M, Sternlieb B, Tsao JC, Zeltzer LK. Yoga for youth in pain: the UCLA pediatric pain program model. Holist Nurs Pract. 2012;26:262–71. doi: 10.1097/HNP.0b013e318263f2ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells RE, Burch R, Paulsen RH, Wayne PM, Houle TT, Loder E. Meditation for migraines: a pilot randomized controlled trial. Headache. 2014;54:1484–95. doi: 10.1111/head.12420. [DOI] [PubMed] [Google Scholar]
- 54.Holroyd KA, Drew JB. Behavioral approaches to the treatment of migraine. Semin Neurol. 2006;26:199–207. doi: 10.1055/s-2006-939920. [DOI] [PubMed] [Google Scholar]