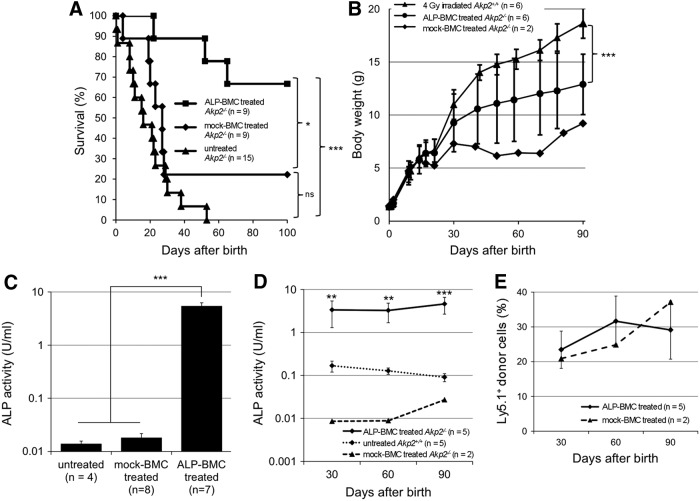
Figure 1.
Therapeutic effects of ex vivo gene therapy in neonatal Akp2−/− mice. (A) Survival curves for ALP-BMC-treated, mock-BMC-treated, and untreated Akp2−/− mice. *p < 0.05, ALP-BMC-treated vs. mock BMC-treated Akp2−/− mice; ***p < 0.001, ALP-BMC-treated vs. untreated Akp2−/− mice; ns, not significant (p = 0.14, mock BMC-treated vs. untreated Akp2−/− mice). (B) Comparison of average body weights among Akp2+/+ mice irradiated at a dose of 4 Gy, ALP-BMC-treated Akp2−/− mice (4 Gy irradiation), and mock-BMC-treated Akp2−/− mice (4 Gy irradiation). ***p < 0.001, ALP-BMC-treated vs. 4 Gy-irradiated Akp2+/+ mice on day 90. (C) Comparison of plasma ALP activity 1 week after treatment among untreated (n = 4), mock-BMC-treated (n = 8), and ALP-BMC-treated (n = 7) Akp2−/− mice. ***p < 0.001 vs. ALP-BMC-treated Akp2−/− mice. (D) Time course of the changes in plasma ALP activity in ALP-BMC-treated Akp2−/− mice, untreated Akp2+/+ mice, and mock-BMC-treated Akp2−/− mice. **p < 0.01, ***p < 0.001, ALP-BMC-treated vs. untreated Akp2+/+ mice. (E) Long-term engraftment of Ly5.1+ donor cells in ALP-BMC-treated and mock-BMC-treated Akp2−/− mice. ALP, alkaline phosphatase; BMC, bone marrow cells.