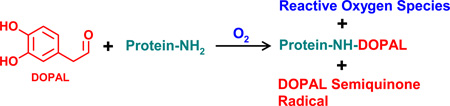
Abstract
3,4-Dihydroxyphenylacetaldehyde (DOPAL) is an endogenously produced toxic aldehyde. It is a bifunctional electrophile implicated in the loss of dopaminergic cells concomitant with Parkinson’s disease and neurodegeneration. DOPAL is known to react with proteins and amino acids such as N-acetyl Lys; oxidation of the catechol moiety to the quinone of DOPAL increases this reactivity. Here we demonstrate the ability of the antioxidants N-acetylcysteine, glutathione, and ascorbic acid to mitigate the reactivity of DOPAL with proteins and amino acids in a dose-dependent fashion. Conversely, Trolox did not lessen the observed reactivity with proteins. Interestingly, use of tricine, a buffer and reducing agent, in these systems also decreased the reactivity of DOPAL with amines, yielding tricine-derived free radical species. Modification of amines with aldehydes typically involves Schiff base chemistry; however, the observance of free radicals suggests that an oxidative step is involved in the reaction of DOPAL with lysine. Furthermore, while Schiff base formation is usually optimal at pH 5, the reaction rate of DOPAL with N-acetyl Lys is negligible at pH 5 and is enhanced under basic conditions (e.g. pH 9). Conditions of high pH are also favorable for catechol auto-oxidation, known to occur for DOPAL. The antioxidant-mediated protection demonstrated here suggests that oxidative stress may impart cellular vulnerability to protein modification by DOPAL. Therefore, depleted antioxidants and increased levels of lipid peroxidation products, known to prevent detoxifying metabolism of DOPAL, may present a survival challenge to dopaminergic cells targeted in Parkinson’s disease.
Keywords: 3,4-dihydroxyphenlyacetaldehyde; protein modification; free radicals; protein cross-linking; antioxidant; aldehyde
Graphical abstract
Introduction
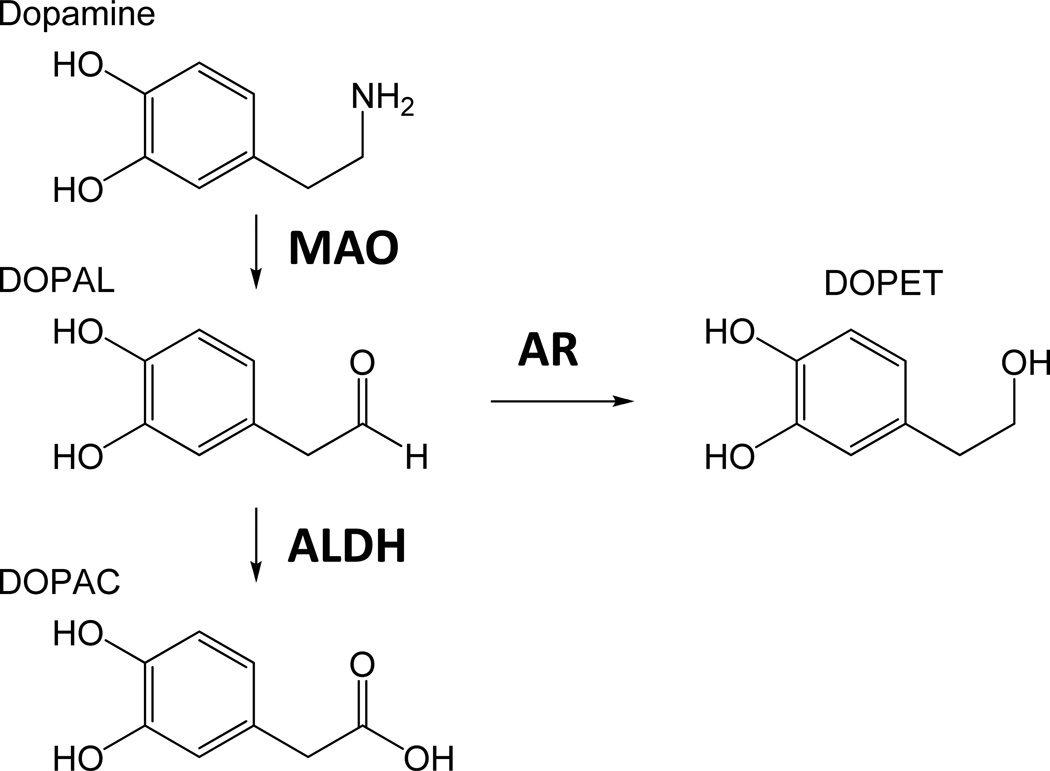
Increased production of several biogenic aldehydes or exposure to environmentally-derived aldehydes is associated with a number of disease states.1, 2 Though a variety of enzymes are capable of detoxifying aldehydes,1 certain metabolic stressors may inhibit these enzymes and lead to elevated cellular levels of toxic compounds.3–6 Metabolism of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine (DA) by monoamine oxidase (MAO) produces an aldehyde intermediate plus hydrogen peroxide (Scheme 1).7 For DA, the MAO-mediated biotransformation yields 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is metabolized to 3,4-dihydroxyphenylacetic acid (DOPAC) by several aldehyde dehydrogenase (ALDH) enzymes. Previous work found DOPAL to be highly toxic; it has been hypothesized that an elevated level of DOPAL is a factor in the loss of dopaminergic neurons associated with Parkinson’s disease (PD).8–11 This “catechol-aldehyde hypothesis” is based on findings that impairments in DA metabolism and trafficking: 1) are observed in PD brains;8, 12 and 2) correlate with selective loss of DA neurons in various animal models, e.g., MAO overexpression, ALDH knockout, decreased expression of vesicular monoamine transporter (VMAT2) and increased expression of the dopamine transporter (DAT).8, 13–16 In addition, recent work correlated exposure of the fungicide benomyl, a potent ALDH inhibitor, to increased risk of PD and suggested ALDH inhibition to be a pathogenic mechanism for PD.17, 18
Scheme 1.
Metabolism of Dopamine.
DOPAL is capable of protein modification, enzyme inhibition, and protein aggregation.2, 19–23 In addition to its toxic potential as an aldehyde, DOPAL possesses a catechol ring that can oxidize to a semiquinone radical (a one-electron oxidation) and an ortho-quinone (a two-electron oxidation). The ortho-quinone derived from DOPAL is a bifunctional electrophile that could potentially aggregate and cross-link proteins.24
Modification of protein by DOPAL is believed to be mediated via formation of a Schiff base with a nucleophile such as a Lys residue.2, 20 Previous work, utilizing mass spectrometry, demonstrated that DOPAL forms adducts with model peptides yielding an adduct of mass consistent with a Schiff base, suggesting protein modification involves the reaction of amines with the aldehyde of DOPAL.20, 25 In addition, replacing DOPAL’s aldehyde/carbonyl group with a nitrile greatly decreased protein reactivity and toxicity compared to DOPAL.26 A similar result occurs when DOPAL is modified chemically to prevent catechol oxidation. O-methylation decreases protein reactivity, and amine reactivity requires sodium cyanoborohydride (NaCNBH3) reduction for adduct stability.20 Interestingly, sodium cyanoborohydride was found to decrease the rate for the reaction of DOPAL with N-acetyl Lys; sodium cyanoborohydride will reduce both Schiff bases and quinones.27 In addition, the 3-O-methyl analog of DOPAL demonstrated greatly diminished toxicity to dopaminergic cells.26 These results suggest that protein modification by DOPAL involves reaction of amines with the aldehyde group; however, catechol oxidation influences DOPAL’s protein reactivity and toxicity.
The catechol of DA auto-oxidizes to a quinone that readily forms adducts with Cys via 1,6-conjugate addition.28–30 Like DA, DOPAL undergoes oxidation to a semiquinone radical and an ortho-quinone,24 and therefore, N-acetyl Cys would be expected to form a similar adduct with DOPAL-quinone. Previous studies have shown that DOPAL is reactive with N-acetyl Lys, but unreactive with N-acetyl Cys at physiological pH.20 However, DOPAL was found to readily crosslink proteins, possibly via a Cys-DOPAL-Lys linkage, and such protein crosslinking could be prevented by ascorbate.20 Such a result suggested the involvement of an ortho-quinone intermediate in protein cross-linking. In an attempt to model protein cross-linking with N-acetyl Lys and N-acetyl Cys, it was discovered that the reaction of DOPAL with protein-type amines requires an oxidative step that produces reactive oxygen species. This unexpected finding was explored further in the current study.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. DOPAL was biosynthesized from MAO harvested from rat liver31 and also synthesized from epinephrine via Fellman’s method.32 DOPAL samples were analyzed for concentration and purity using an Agilent 1200 Capillary HPLC equipped with a photo-diode array detector. Separation was performed using a Phenomenex Luna C18 (1 mm × 150 mm, 100 Å) column running mobile phase 0.1% TFA (v/v) in H2O at 94% and ACN at 6%, with a flow rate of 50 µL/min. Absorbance of DOPAL (retention time ≈7.5 min) was measured at 280 and 202 nm, and concentration was determined by comparison with previously analyzed standard curves.5
Evaluation of DOPAL Reactivity
To determine the reactivity of DOPAL in the presence of both Lys and Cys residues, samples containing 100 µM DOPAL and 10 mM N-acetyl Lys (100x eq compared to the number of equivalents (eq) of DOPAL; eq given below are all relative to the amount of DOPAL) were incubated in 50 mM sodium phosphate buffer, pH 7.4 for 2 h at 37 °C in the dark. A negative control, DOPAL with no N-acetyl Lys, was run with each experiment to subtract background loss of DOPAL e.g., oxidation. Reactivity was determined by a decrease in measured DOPAL concentration. Aliquots were taken for HPLC analysis at 30 min intervals and the reaction ended by a 1:1 dilution with 2% TFA in H2O. Analysis was performed by HPLC as described above, using 5 µL injections unless noted otherwise. This assay was then repeated with 10x eq of N-acetyl Cys (1.00 mM). Based on the results, the assay was performed again with 10x eq (1.00 mM) of ascorbic acid, which exists predominately as the ascorbate monoanion (AscH−) at pH 7.4. These experiments were then repeated using 3-h incubations and a range of concentrations of reducing agents: 0, 10, 50, 100, 200, and 1000 µM N-acetyl Cys (0, 0.1x, 0.5x, 1x, 2x and 10x eq) and 0, 50, 100, and 200 µM (0, 0.5x, 1x, and 2x eq) AscH−. Samples were collected every 30 min and analyzed as above.
Similarly, the impact of pH on DOPAL reactivity was evaluated by incubating 100 µM DOPAL and 10 mM N-acetyl Lys for 2 h in 50 mM sodium phosphate buffer over a range of pH values (5.0, 6.0, 7.0, 7.4, 8.0 and 9.0). Aliquots were taken at 30 min intervals, diluted with acid, and analyzed by HPLC as described above. Assays repeated without N-acetyl Lys served as a control for each experiment to account for spontaneous loss of DOPAL at the respective pH values.
Finally, reactivity was assessed in the presence of a radical scavenging buffer, tricine.33 For this assay, 100 µM DOPAL and 10 mM N-acetyl Lys were incubated in the dark for 2 h at 37 °C using either 50 mM sodium phosphate, pH 7.4 or 50 mM tricine pH 7.4 buffer. Aliquots were taken at 0, 1, and 2 h for HPLC analysis. Assays without N-acetyl Lys served as controls. Reactions were ended by 1:1 dilution with 2% aqueous TFA to quench the reaction, and analyzed by HPLC. Statistical analyses were conducted using GraphPad Prizm 4.0 (GraphPad Software, San Diego, CA).
Analysis of DOPAL Reactivity by Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) was used to investigate the structure and oxidative potential of species formed by the reaction of DOPAL with N-acetyl Lys. This experiment involved incubating 500 µM DOPAL in 25 mM tricine pH 7.4 and 0.5 M MgCl2 with 50 mM N-acetyl Lys (10x eq) at 37 °C for 2 h followed by EPR analysis. DOPAL in tricine buffer and N-acetyl Lys in tricine buffer served as controls. Note that all buffer solutions used distilled and deionized water that had been treated with Chelex 100 to remove redox active metals.
EPR investigations utilized a Bruker EMX spectrometer with an Aqua-X sample holder and an ER 4119HS cavity. Spectra were recorded at ambient temperature using 1.0 mL sample volumes. Typical EPR parameters were: 3510.75 G center field; 24 G sweep width; 9.854 GHz microwave frequency: 20 mW nominal power; 2.52 × 104 receiver gain: 100 kHz modulation frequency; 0.20 G modulation amplitude; 40.96 ms conversion time: 81.92 ms time constant: 41.92 s sweep time: and up to 10 X-scans for each 1024 point spectrum. Spectra shown are representative of at least three trials.
Impact of Antioxidant Treatment on DOPAL-Protein Modification
To examine the effect of antioxidant treatment on the ability of DOPAL to modify a model protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was incubated at a final concentration of 0.3 mg/mL (8.3 µM) with 5 mM antioxidant and 50 µM DOPAL in 50 mM sodium phosphate buffer, pH 7.4 at 37 °C for 4 h. Incubations lacking DOPAL served as controls. GAPDH is a relevant model as it readily aggregates/cross-links in the presence of DOPAL, and is important to viability of DA neurons.34 Samples were frozen and stored at −20 °C for subsequent analysis, which involved separating 9 µg of protein on 10% SDS-PAGE gels, staining with Coomassie and densitometry measurements using NIH ImageJ 1.44p. Tested antioxidants included N-acetyl Cys, glutathione (GSH), ascorbic acid, as ascorbate monoanion (AscH−) because the pH was typically 7.4, sodium cyanoborohydride (NaCNBH3), and Trolox. Trolox was dissolved in DMSO. AscH− was prepared in 50 mM sodium phosphate buffer, pH 7.4 using crystalline ascorbic acid, and all other antioxidants were dissolved in water. The density of the 37 kDa monomer band was compared between samples. Reduction of band density and formation of higher molecular weight species was used to indicate protein modification and aggregation. Statistical analyses were performed using GraphPad Prizm 4.0.
Based on these results, select reducing agents/antioxidants (N-acetyl Cys, GSH, AscH−) were then investigated across a range of concentrations. Incubations utilized 0.3 mg/mL GAPDH with 50 µM DOPAL in 50 mM sodium phosphate pH 7.4 and 0, 5, 25, 50, 100, or 500 µM antioxidant. The same conditions were used as described above.
Protein samples were then analyzed for bound DOPAL using nitroblue tetrazolium (NBT), a dye that stains indigo in the presence of redox active functional groups such as catechols or quinones.26 As above, 9 µg samples of GAPDH were separated on 10% SDS-PAGE gels. The separated proteins were then transferred to nitrocellulose using a Trans-Blot-SD (BioRad, Hercules, CA) semidry transfer apparatus at 20 V for 45 min. The membrane was then immersed in 0.24 mM NBT in 2 M potassium glycinate pH 10 for 48 h.
Finally, these samples were examined for protein aggregation. Samples of 1 µg GAPDH, prepared with DOPAL as previously described, were separated on a 10% SDS-PAGE, then transferred as described above. The membrane was blocked in 5% BSA in TBS-T. Primary antibody, Rabbit anti-GAPDH (Sigma-Aldrich, St Louis, Mo), was applied at 1:10000. Secondary antibody, Goat anti-Rabbit-HRP (Santa Cruz Biotechnology, Santa Cruz, CA) was applied at 1:10000. The membrane was developed using ECL plus a Chemiluminescence kit (GE Healthcare, Piscataway, NJ) and detected using Hyperfilm-ECL (GE Healthcare, Piscataway, NJ). The films were analyzed using NIH Image J.
Reactivity of DOPAL with GAPDH in the Presence of a Radical Scavenger
To determine the impact of a radical scavenger on DOPAL reactivity with protein, the GAPDH aggregation experiments were repeated as above using tricine buffer. GAPDH (0.3 mg/mL) was incubated for 4 h at 37 °C with 50 µM DOPAL in 50 mM sodium phosphate buffer pH 7.4 or 50 mM tricine pH 7.4. Omitting DOPAL served as controls for these experiments. 9 µg of protein were separated on 10% SDS-PAGE gel, stained with Coomassie and analyzed using ImageJ 1.44p. Statistical analysis was performed using GraphPad Prizm 4.0.
Results
DOPAL reactivity with amines decreases in the presence of antioxidants
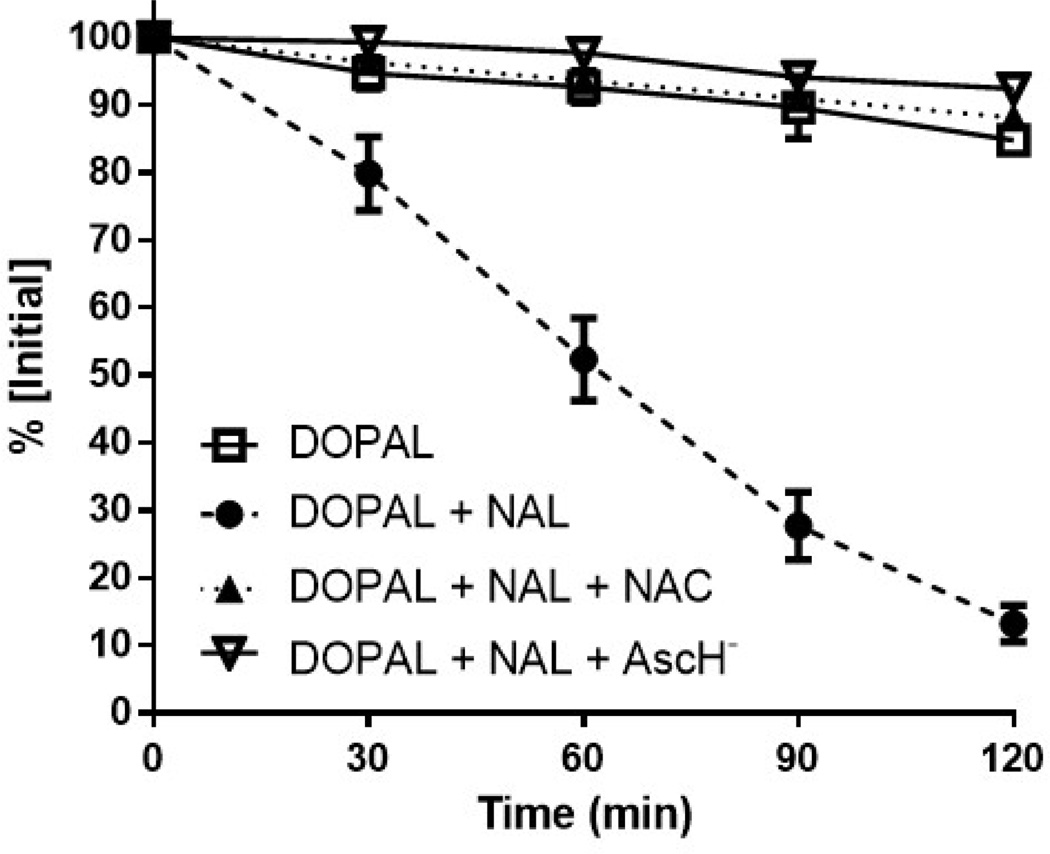
DOPAL reactivity with N-acetyl Lys in the presence of N-acetyl Cys was determined by measuring decreases in DOPAL concentration over time by HPLC. Figure 1 demonstrates baseline loss of DOPAL without N-acetyl Lys. At 2 h, control DOPAL levels decreased to 85% of original. When DOPAL was incubated with N-acetyl Lys, much greater loss was apparent; the concentration of DOPAL dropped to 13% of initial. In the presence of 10 eq of N-acetyl Cys, DOPAL levels only dropped to 88%. Similarly, DOPAL levels decreased to only 93% when 10 eq of AscH− were included. Interestingly, samples including either N-acetyl Cys or AscH− demonstrated less loss of DOPAL, 12% and 7% loss respectively (data not shown), as compared to the 15% loss in the untreated condition.
Figure 1.
The reactivity of DOPAL with NAL is greatly diminished in the presence of the antioxidants NAC and AscH− (n = 4) over time (min). %[Initial] is calculated as the % remaining DOPAL compared to the concentration at time zero. NAC and AscH− were used at 1 mM, DOPAL at 100 µM, and NAL 10 mM.
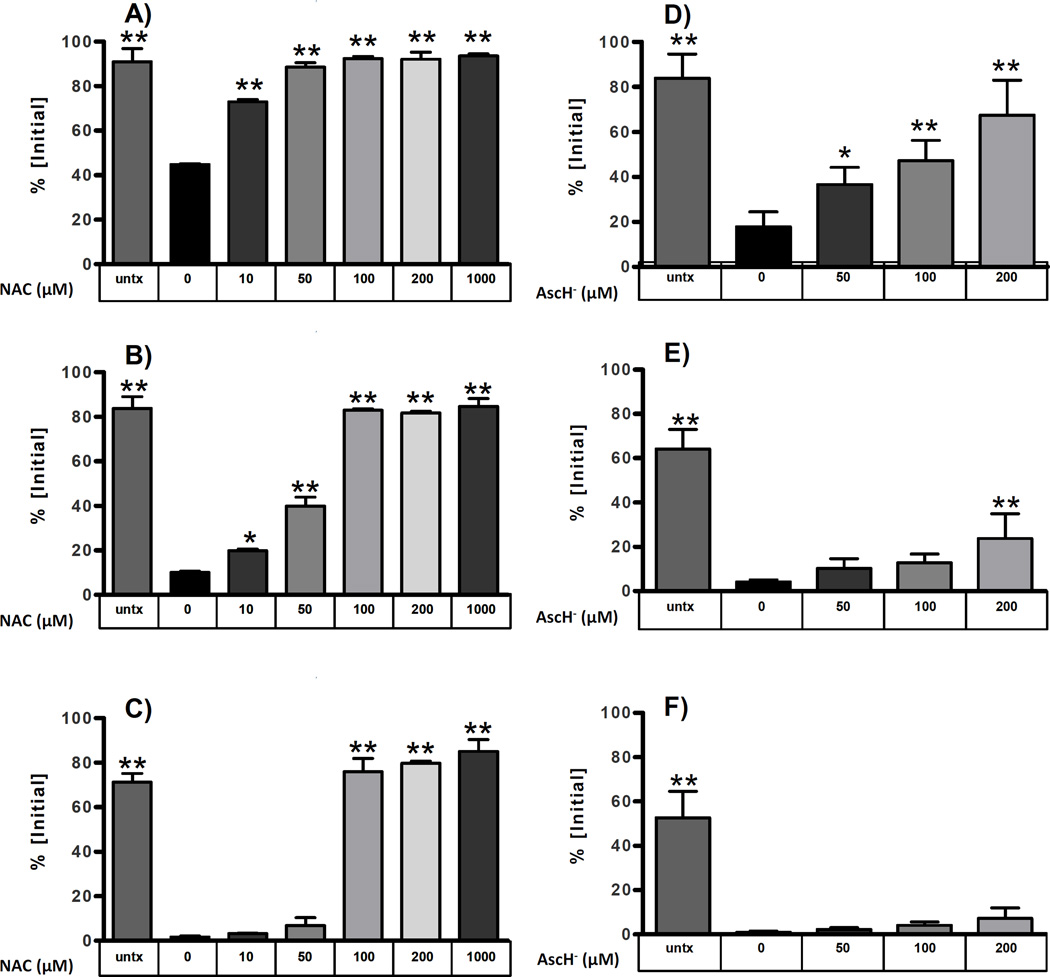
To further investigate the protective effects of antioxidants on DOPAL reactivity, 3-h incubations were performed utilizing a range of N-acetyl Cys and AscH− concentrations. Both antioxidants demonstrated a dose-dependent reduction in DOPAL loss (Figure 2). At one hour, the observable loss of DOPAL with N-acetyl Cys is minimal, even at only 50 µM (Figure 2A). Although AscH− had less of a protective effect than N-acetyl Cys at 1 h, all AscH− treated samples maintained a significantly higher DOPAL concentration than the DOPAL/N-acetyl Lys control (Figure 2D).
Figure 2.
The antioxidants NAC and AscH- inhibit the reaction of DOPAL with NAL in a concentration-dependent manner. Reaction of 100 µM DOPAL with 10 mM NAL was attenuated by NAC, as shown at: (A) 1 h; (B) 2 h; and (C) 3 h. In addition, AscH- inhibited the reaction of 100 µM DOPAL with 10 mM NAL as shown at: (D) 1 h; (E) 2 h and (F) 3 h. Results are calculated as a percentage of the DOPAL concentration at time 0, and differences were determined by one way ANOVA with Neuman Keuls Multiple comparison post test *, p<0.01 and **, p<0.001.
At 2 h, higher concentrations of N-acetyl Cys (≥ 100 µM) continue to demonstrate a negligible loss of DOPAL through oxidation or reaction with N-acetyl Lys (Figure 2B). The 10 and 50 µM samples, however, display a marked increase in DOPAL loss. Nevertheless, these samples maintain a DOPAL concentration significantly greater than the DOPAL/N-acetyl Lys control. In the AscH−-treated samples at 2 h, a substantial loss of DOPAL is observed. However, DOPAL levels remain well above control levels, and the loss continues to follow a concentration dependent pattern (Figure 2E).
After 3 h, levels of DOPAL in the control sample (without N-acetyl Lys) fell to 70% of the initial concentration (Figure 2C). Samples with 100 µM (1x eq) or higher N-acetyl Cys showed the smallest decrease of DOPAL. The DOPAL concentration of the 10 mM N-acetyl Lys sample (without N-acetyl Cys) dropped to less than 2% of the original concentration. While samples treated with low concentrations of N-acetyl Cys showed only slightly higher DOPAL levels than the DOPAL/N-acetyl Lys control, the higher concentrations of N-acetyl Cys (1x, 2x, and 10x eq) exhibited DOPAL concentrations greater than 75% of initial concentration. Compared to the DOPAL/N-acetyl Lys sample and negative control (DOPAL in buffer), the decrease in DOPAL concentration was much less when N-acetyl Cys was included in incubations. This was a significantly smaller reduction in DOPAL than observed in the DOPAL/N-acetyl Lys control, and DOPAL levels were even higher than in the DOPAL control. Though AscH− produced a smaller DOPAL loss at 1000 µM (10x eq) than N-acetyl Cys at the early time point, it did not prove effective at lower concentrations. By 3 h, DOPAL concentrations in all AscH− samples had fallen below 10% of initial levels (Figure 2F).
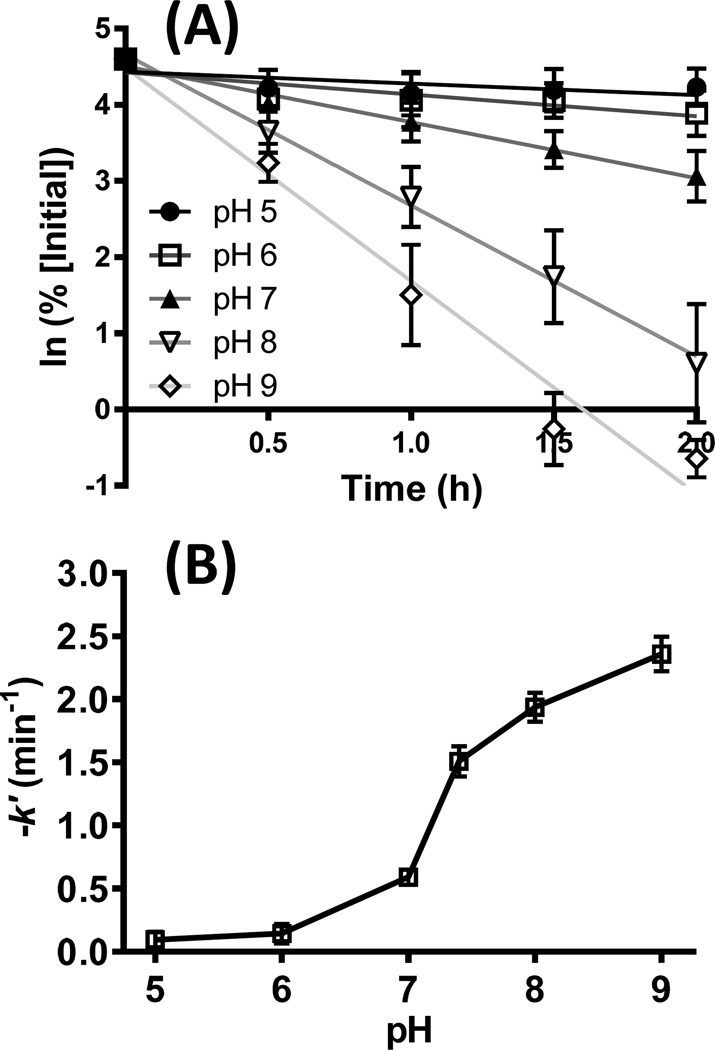
pH dependence of DOPAL reactivity with amines
As buffer pH increased, the rate of spontaneous DOPAL loss increased as well (data not shown). Similarly, the rate of DOPAL reactivity with N-acetyl Lys was greater at higher pH (Figure 3A and 3B). Graphing the negative of the pseudo-first order rate constant of DOPAL reactivity for N-acetyl Lys (−k’) vs. pH yielded a sigmoidal-shaped curve (Figure 3B).
Figure 3.
Reactivity of DOPAL with NAL increases with pH. 100 µM DOPAL incubated with 10 mM NAL for 2 h in 50 mM Na2PO4 at pH 5, 6, 7, 7.4, 8, and 9 displays increased loss of DOPAL with increasing pH. Percent initial calculated as individual sample concentration relative to T = 0 concentration (n = 4). (A) Reaction time course demonstrating pH dependence for DOPAL reactivity with NAL. (B) The pseudo-first order rate constant (k') for the reaction of DOPAL with 10 mM NAL increases with pH.
The reactivity of DOPAL decreases in the presence of a radical scavenger
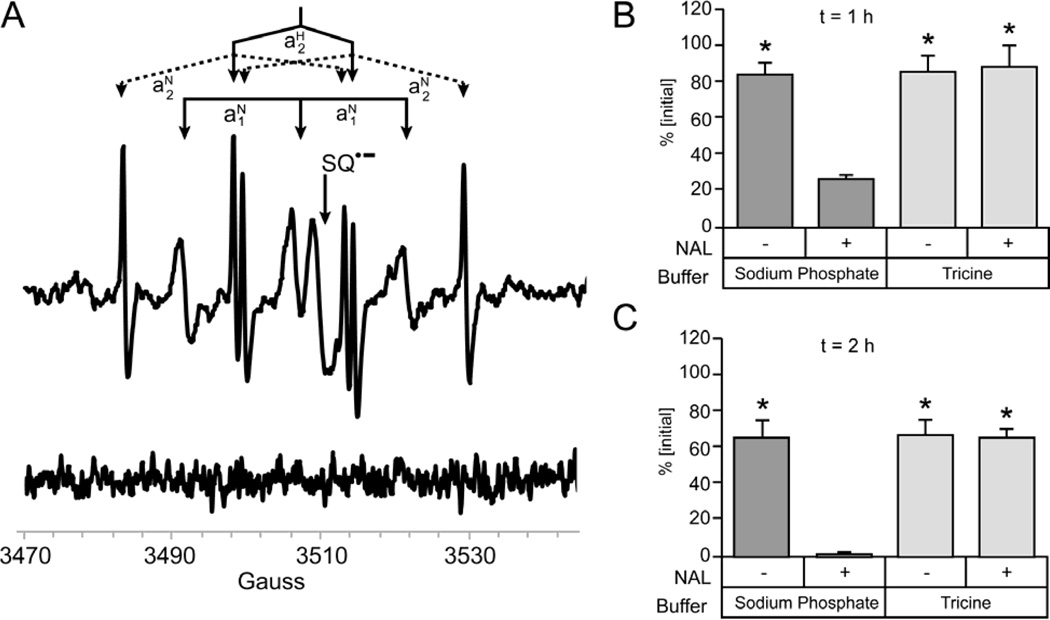
EPR spectroscopy was used to detect free radicals formed during the reaction between N-acetyl Lys and DOPAL. Previous work has shown that the oxidation of DOPAL is important for reactivity;20 DOPAL auto-oxidation proceeds through the formation of a DOPAL semiquinone radical.24 If formed, the Schiff-base radical would very likely demonstrate similar splitting patterns to the aforementioned DOPAL radical. However, when 400 µM DOPAL was incubated with 100 eq of N-acetyl Lys in tricine pH 7.4 for 2 h, the expected splitting patterns were absent. Instead, two nitroxide free radicals derived from tricine (radical-1, g1 = 2.006, aN1 = 14.7 G,; radical-2, g2 = 2.006, aN2 = 14.7 G, aH2 = 15.7 G) were detected as well as a semiquinone radical (g = 2.004) (Figure 4A). The tricine-derived radicals only formed when DOPAL and N-acetyl Lys were incubated together. Either component alone did not produce a detectable level of radical (Figure 4A). Also, eliminating the 2-h incubation time did not allow detectable levels of free radicals to be formed (data not shown).
Figure 4.
Tricine scavenges radicals from the reaction of DOPAL and NAL. (A) The upper EPR spectrum results from the incubation of 40 mM NAL and 400 µM DOPAL. Three species are present, a semiquinone radical (g = 2.004) and two nitroxide free radicals derived from tricine (radical-1, g1 = 2.006, aN1 = 14.7 G; radical–2, g2 = 2.006, aN2 = 14.7 G aH2 = 15.7 G;). A previous study on free radicals derived from tricine reports these same two nitroxide free radicals and, upon correction, have identical hyperfine splittings.33 Note that these authors made a minor error in determining hyperfine splittings. The lower spectrum shows that 40 mM NAL incubated without DOPAL produced no detectable free radicals. (B) When 0.1 mM DOPAL and 10 mM NAL are incubated in 50 mM tricine buffer pH 7.4 a decreased rate of loss of DOPAL is seen compared to DOPAL and NAL incubated in 50 mM Na2PO4 pH 7.4 at 1 h and (C) at 2 h (n = 3). %[Initial] calculated as individual sample concentration relative to t = 0 concentration. Significance was determined by one-way ANOVA with Neuman Keuls Multiple comparison post test * = p < 0.001.
In order to investigate the potential radical scavenging effects of tricine on the DOPAL/N-acetyl Lys reaction, 2-h incubations of DOPAL (100 µM) with or without N-acetyl Lys (10.0 mM) were carried out in either 50 mM sodium phosphate pH 7.4 or 50 mM tricine pH 7.4 buffers. In phosphate buffer, the N-acetyl Lys reaction showed DOPAL losses of 73% and 98% at 1 and 2 h, respectively (Figure 4B and 4C). Conversely, in tricine buffer DOPAL losses were minimal.
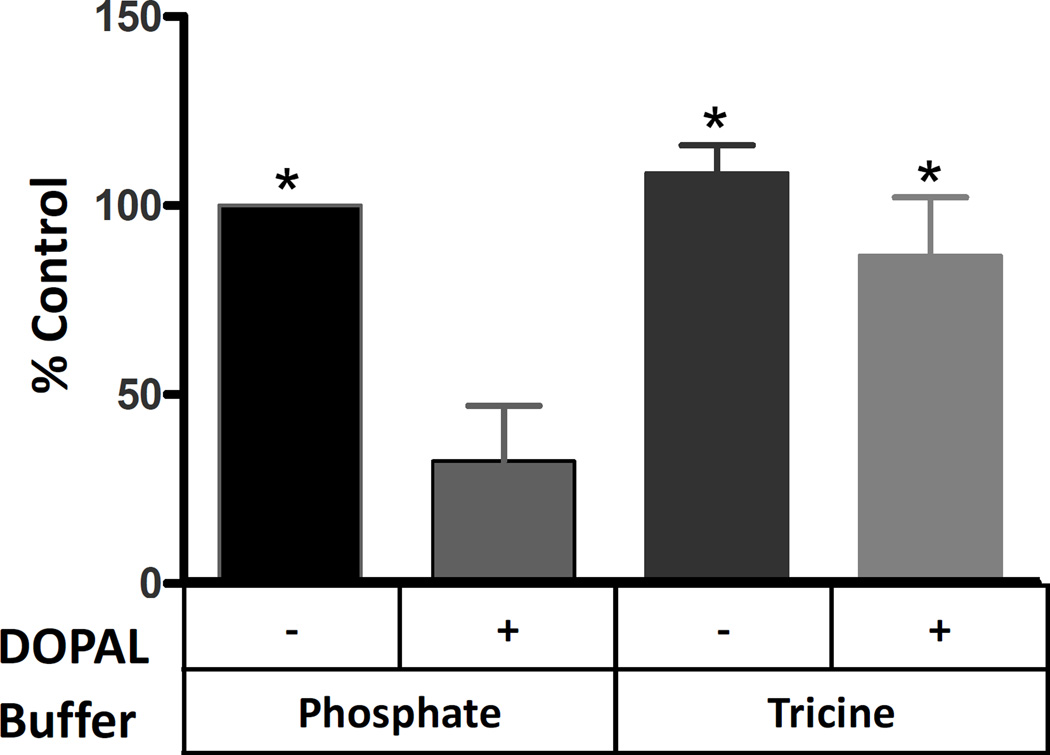
Previous studies have demonstrated DOPAL’s ability to mediate protein cross-linking and aggregation.20 The effect of a radical scavenging buffer on such a reaction was investigated. GAPDH (0.3 mg/mL) was incubated with or without DOPAL (50 µM) in either 50 mM phosphate buffer pH 7.4 or 50 mM tricine buffer pH 7.4. Incubation of DOPAL and GAPDH in phosphate buffer resulted in a 68% loss of density in the 37 kDa parent band as compared to untreated control (Figure 5). DOPAL and GAPDH in tricine buffer displayed only a 23% loss of the 37 kDa parent band compared to untreated control. This corresponds to a 45% reduction of DOPAL reactivity in tricine buffer. Although the loss of density for the DOPAL treated sample in tricine was significant with respect to the untreated GAPDH in tricine, neither tricine sample was significantly different from the untreated phosphate buffer control.
Figure 5.
GAPDH incubated with 50 µM DOPAL in 50 mM Na2PO4 buffer pH 7.4 for 4 h showed a substantial decrease in band density. GAPDH incubated with 50 µM DOPAL in 50 mM tricine buffer pH 7.4 for 4 h displayed some loss of band density, but was significantly greater density than GAPDH with DOPAL in phosphate. % Control refers to the integrated staining density of the GAPDH monomer relative to untreated sample. Significance was determined by one way ANOVA with Neuman Keuls Multiple comparison post test * p < 0.001. n = 6.
Antioxidant treatment reduces protein modification and aggregation by DOPAL
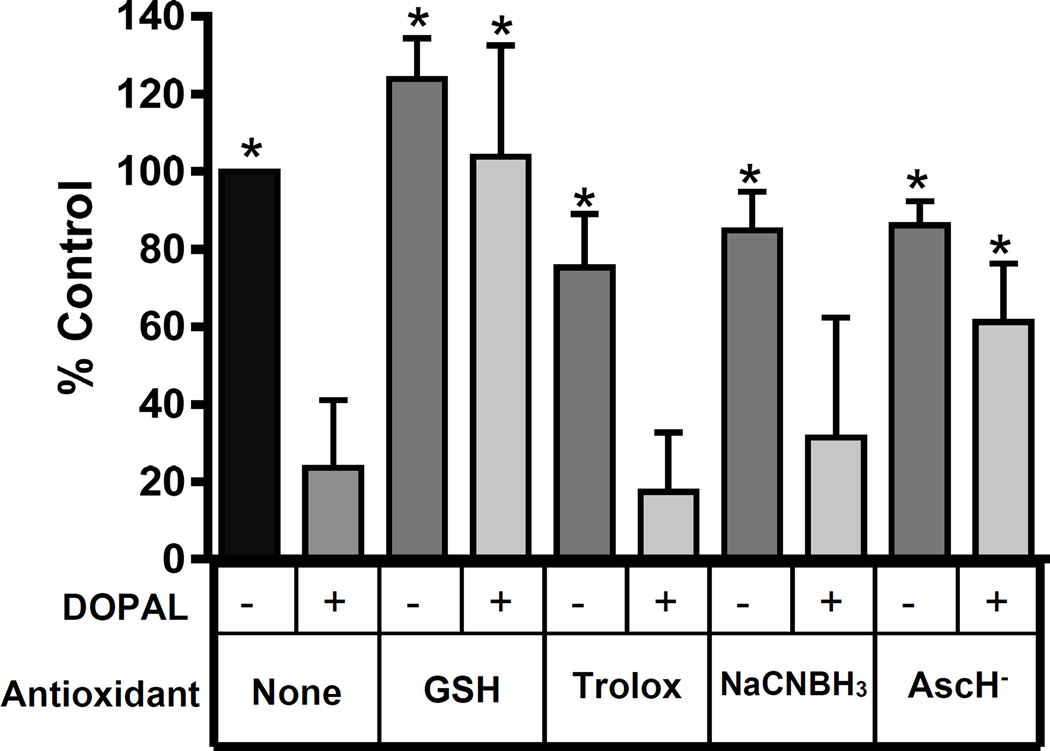
Data in the previous sections demonstrate reactivity of DOPAL towards a protein nucleophile (NAL) in the presence and absence of antioxidants; however, we wanted to know if antioxidants modulated reactivity of DOPAL towards a protein, which is composed of numerous nucleophiles. To explore the impact of reducing agents/antioxidants on DOPAL reactivity with a model protein, GAPDH (0.3 mg/mL) and DOPAL (50 µM) were incubated in the presence of a variety of antioxidants (5 mM). Densitometry results are displayed in Figure 6. Incubating GAPDH and DOPAL without antioxidant resulted in a significant loss of the 37 kDa band density; decreasing to 24% of the untreated control. AscH− and GSH had a protective effect. AscH− and GSH treatment yielded GAPDH band densities of 61% and 103%, respectively, compared to the untreated control. NaCNBH3 or Trolox did not significantly mitigate loss of GAPDH band density due to DOPAL, showing 31% and 17% of control, respectively. Antioxidant control incubations with protein but not DOPAL were not significantly different from the untreated GAPDH control. However, NaCNBH3, AscH−, and Trolox treated samples all demonstrated a slight loss of 37 kDa band density while GSH treated GAPDH showed an increase in band density.
Figure 6.
Antioxidants attenuate reactivity of GAPDH with DOPAL. % Control refers to the integrated staining density of the GAPDH monomer relative to untreated sample. All incubation times were 4 h versions (0.3 mg/mL GAPDH, 50 µM DOPAL, and 5 mM antioxidant in 50 mM sodium phosphate buffer, pH 7.4, 37 °C). GAPDH alone was significantly different from GAPDH with DOPAL (n = 13) as determined by one way ANOVA with Neuman Keuls multiple comparison post test (* = p < 0.001). GAPDH with antioxidants were all also significantly different from GAPDH with DOPAL. GAPDH and DOPAL with GSH (n = 7) or with AscH− (n = 6) were significantly different from GAPDH with DOPAL. GAPDH and DOPAL with Trolox or with NaCNBH3 showed no significant difference from GAPDH and DOPAL.
Based on these results, GSH and AscH− were investigated for their ability to prevent GAPDH protein aggregation over a range of concentrations. N-acetyl Cys was similarly investigated, as it was previously shown to diminish DOPAL reactivity with N-acetyl Lys.
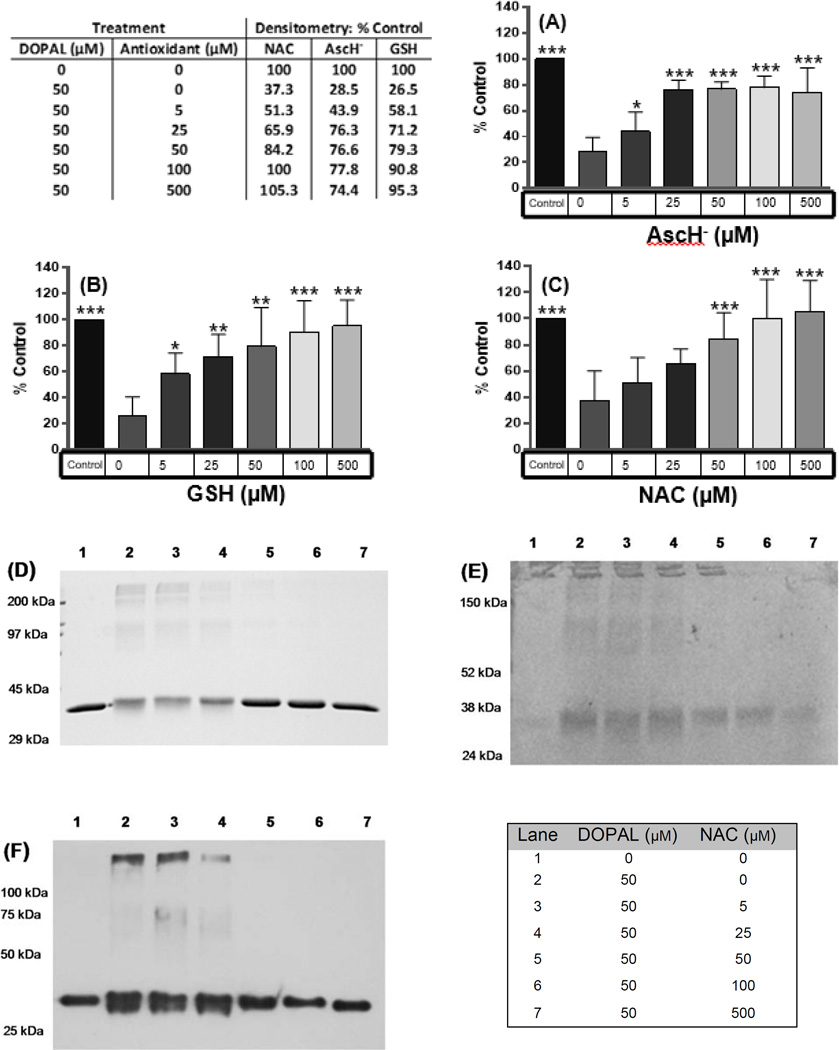
These experiments utilized the same conditions previously applied to GAPDH, and DOPAL mediated protein aggregation was measured in the same fashion. While treatment of GAPDH with DOPAL resulted in a significant loss of the 37 kDa band density, antioxidants prevented loss of the 37 kDa band density in a dose-dependent manner (Figure 7). All antioxidants tested resulted in noticeable positive changes in band density starting at 0.1x eq (i.e., 5 µM; 1x eq is 50 µM, the concentration of DOPAL used in this set of experiments), but the increase was only significant for GSH and AscH− (Figure 7A and 7B) at that level. Mitigation of the loss of 37 kDa band density with N-acetyl Cys was not significant until 1.0x eq of antioxidant was used (Figure 7C and 7D). Also, N-acetyl Cys was the only antioxidant that completely prevented aggregation/cross-linking of GAPDH. Ultimately, GSH and AscH− afforded 95% and 74% protection of protein aggregation/cross-linking, respectively. AscH− appeared to reach maximum protective effect at 0.5x eq, while the protection afforded by N-acetyl Cys and GSH continued to increase as with their concentrations.
Figure 7.
The antioxidants: (A) AscH−, (B) GSH, and (C) NAC inhibited DOPAL’s protein reactivity in a concentration dependent fashion. Loss of GAPDH monomer band density after a 4-h incubation with DOPAL at 37 °C (0.3 mg/mL GAPDH, 50 µM DOPAL in sodium phosphate buffer, pH 7.4). % Control refers to the integrated staining density of the GAPDH monomer relative to untreated sample. The table shows the average density with each treatment, NAC n = 5, AscH− n = 6, and GSH n = 5. Significance with respect to GAPDH incubated with 50 µM DOPAL was determined by one way ANOVA with Neuman Keuls Multiple comparison post test (* =p<0.05, **= p< 0.01, and *** =p<0.001). All treatments exhibit increased band density as antioxidant concentration increases. Example of data demonstrating antioxidants protect protein against DOPAL-mediated modification and aggregation. (D) Coomassie, (E) NBT, and (F) Western blot analysis demonstrate attenuation of DOPAL-mediated GAPDH aggregation by antioxidants. GAPDH was treated with 50 µM DOPAL for 4 h at 37 °C in 50 mM Na2HPO4 buffer, pH 7.4. Lane 1, no DOPAL; Lane 2, 50 µM DOPAL; Lanes 3–7, 50 µM DOPAL with 5, 25, 50, 100 and 500 µM NAC. The 37 kDa band represents monomeric GAPDH.
Modification of GAPDH by DOPAL was then further examined using NBT staining. NBT produces purple staining in the presence of redox active compounds such as DOPAL. Representative results for N-acetyl Cys are shown in Figure 7, and similar results were obtained for GSH and AscH− (data not shown). Figure 7D is a Coomassie stained gel, analyzed above. Figure 7E displays the samples reanalyzed using NBT staining. As expected, no NBT staining was noted in untreated control samples (lane 1). The DOPAL-treated sample (lane 2) displayed NBT staining of both the 37 kDa parent band, and at higher molecular weights, indicating that both GAPDH and aggregates were modified by DOPAL. Also, DOPAL treated lanes displayed broadening of the 37 kDa band in both Coomassie and NBT stained gels. This is another indicator of protein modification. Treatment with a range of antioxidant concentrations (lanes 3–7; 5–500 µM N-acetyl Cys) resulted in a dose dependent reduction of staining for the 37 kDa band and the higher molecular weight aggregates. Broadening of the parent band was similarly reduced. These results indicate that antioxidant treatment results in a decrease in protein modification by DOPAL.
The GAPDH samples were re-analyzed for protein aggregation by Western Blot. GAPDH (1.0 µg) was separated by SDS-PAGE, transferred to nitrocellulose and probed for GAPDH using antibodies providing greater sensitivity to changes in protein aggregation. Western Blot results (samples identical to 7D and 7E) are shown in Figure 7F. Untreated protein (lane 1) displayed a single band at 37 kDa. Treatment with 50 µM DOPAL (lane 2) results in broadening of the parent band and appearance of higher molecular weight species, both indicative of protein modification and aggregation. Treatment with N-acetyl Cys (lanes 3–7) causes a dose-dependent decrease in formation of high molecular weight species and broadening of the parent band. Coomassie, NBT, and Western Blot results indicate a concentration dependent decrease in GAPDH modification and aggregation by DOPAL when treated with antioxidants.
Discussion
While the mechanism of protein modification by DA is well established, there is much less known about protein reactivity with DOPAL. DA undergoes catechol auto-oxidation to an ortho-quinone that rapidly reacts with thiols (e.g., Cys) via 1,6-conjugate addition.35 The ability of DA quinone to modify proteins by conjugate addition is frequently used as an indicator of increased DA levels in the brain.30 Similarly, the DA metabolite, DOPAC also oxidizes to a quinone and produces a cysteinyl conjugate.30 As DOPAL is known to undergo catechol oxidation to a quinone,24 protein modification was expected to occur via conjugate addition with strong nucleophiles such as sulfhydryl groups on Cys residues, as is known with DA quinone. However, we previously demonstrated that DOPAL was not reactive with N-acetyl Cys at physiologic pH.20
DOPAL has previously been shown to react with N-acetyl Lys and readily causes protein cross-linking and aggregation.20 The latter result suggests that DOPAL is a bifunctional electrophile and that protein modification involves both Schiff base formation with Lys and conjugation with Cys. However, the apparent lack of reactivity between DOPAL and N-acetyl Cys suggested that Schiff-base formation preceded conjugate addition, potentially enhancing the reactivity or oxidation potential of the catechol.
In an attempt to model protein cross-linking, DOPAL was reacted with N-acetyl Lys and N-acetyl Cys at varying concentrations. If Schiff base formation enhanced catechol reactivity and activated 1,6-conjugate addition, the addition of N-acetyl Cys to the reaction mixture was expected to increase the loss of DOPAL. Unexpectedly, Cys was discovered to inhibit the modification of Lys by DOPAL. It is unlikely that N-acetyl Cys reacts with the aldehyde of DOPAL to form a thiohemiacetal; thiohemiacetals are generally unstable and require high levels of thiols for favorable equilibrium of formation, such as in the case for the reaction of GSH with formaldehyde.36 In addition, ascorbate, which has no thiol, protected DOPAL from reaction with protein amines and N-acetyl Lys. Sodium cyanoborohydride also reduced the rate of amine modification by DOPAL.
Antioxidants blocked reactivity of DOPAL toward protein nucleophiles for both NAL and the model protein GAPDH. In the case of GAPDH, the antioxidants prevented protein reactivity, which also blocked protein cross-linking.
Therefore, antioxidants likely function by inhibiting or reversing catechol oxidation, thereby preventing the reactivity of biogenic aldehydes such as DOPAL with proteins. Similar reductions in DOPAL reactivity were observed using antioxidants (N-acetyl Cys, NaCNBH3, and AscH−), as well as tricine, a known oxy-radical scavenger.33 This indicates that thiohemiacetal formation is not a likely explanation for the protective effect of N-acetyl Cys against DOPAL loss. Furthermore, the noted impact of tricine indicates that radical formation is important in the reaction between DOPAL and N-acetyl Lys.
Interestingly, while NAC and GSH as well as AscH− prevented reaction of DOPAL with NAL and GAPDH, the thiol antioxidants appeared to be efficacious than AscH−. For experiments involving NAL, a 1:1 stoichiometry of NAC:DOPAL completely inhibited the reaction of NAL and DOPAL while a 2:1 ratio of AscH−:DOPAL did not prevent interaction of NAL and DOPAL. In addition, NAC and GSH provided complete protection against DOPAL-mediated GAPDH aggregation; however, even at high AscH−:DOPAL ratios, complete protection against DOPAL-mediated GAPDH aggregation could not be achieved via AscH−. Such findings support the proposed production of reactive oxygen species during the reaction of DOPAL with proteins, and the differential activity of antioxidants (i.e., thiol versus AscH−) suggests unique species of reactive oxygen species are generated. Previous work has demonstrated that NAC is a better scavenger of reactive oxygen species such as hydroxyl radical but lower reactivity for hydrogen peroxide and little if any with superoxide anion;37, 38 however, AscH− was found to be an effective antioxidant to react with superoxide anion.39 Future work will seek to elucidate the mechanism via which different antioxidants variably inhibit DOPAL reactivity with proteins.
The observation that antioxidants block the reaction of DOPAL and other biogenic aldehydes formed from neurotransmitters with proteins was first noted by Ungar et al.19 who demonstrated that AscH−, Cys, GSH and pargyline all inhibited binding of radiolabeled DA to proteins in tissue homogenate. The effectiveness of pargyline as an MAO inhibitor indicated that a DA metabolite was the modifying agent as opposed to DA or DA-quinone. As previously discussed, these agents (especially Cys and GSH) were thought to form thiohemiacetals or otherwise directly interact with DOPAL.40 However, inhibition of protein binding by AscH− sheds some doubt on this theory given AscH− does not contain any nucleophiles.
Interestingly, pH is also a factor in DOPAL reactivity with N-acetyl Lys. Schiff base formation is optimal at approximately pH 5 and typically decreases with increasing pH. An acidic pH is required for the dehydration of the carbinolamine to form the imine.41 The reactivity of DOPAL with N-acetyl Lys remained low at pH 5, but increased with pH. Although the reactivity of DOPAL with N-acetyl Lys is typically believed to involve a Schiff base, the increase in reactivity with pH appears to contradict this hypothesis. The rate of catechol auto-oxidation positively correlates with pH42 and therefore, increasing the pH may enhance the oxidation rate for the DOPAL catechol and thereby augment amine modification by DOPAL.
The DOPAL-protein/peptide adduct does not require reduction for stability, which is atypical for a Schiff base.20, 43 Furthermore, the addition of sodium cyanoborohydride to the reaction of DOPAL with N-acetyl Lys appears to slow down the rate of amine modification.
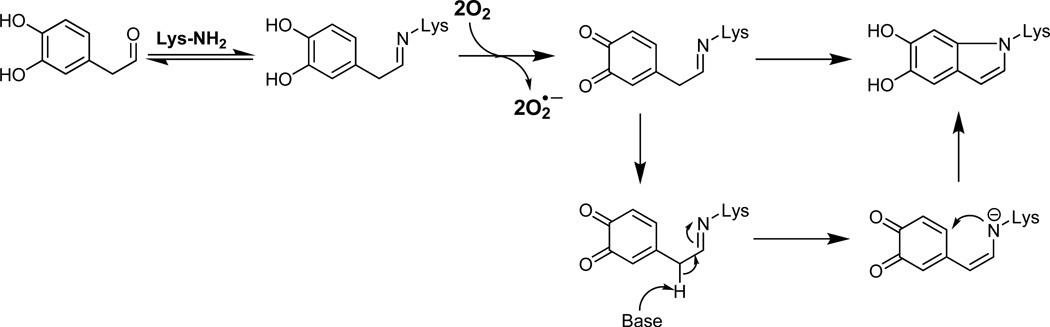
The structure of the protein adduct of DOPAL is unclear but must account for several observations: 1) a lack of nucleophilic reactivity with Cys at physiologic pH, 2) sensitivity of protein modification to antioxidants, 3) pH dependence profile and 4) unusual adduct stability for a Schiff base. In addition, the N-acetyl-Lys conjugate of DOPAL has an indole-like UV profile as seen via HPLC-PDA analysis (data not shown). One possible solution is shown in Scheme 2 and involves reaction of DOPAL with Lys followed by catechol oxidation and subsequent rearrangement to a more stable indole-structure. DA and DOPAC can rapidly cyclize after oxidation to quinones,29 so cyclization may be involved in DOPAL protein modification as also noted in Scheme 2. Unfortunately, our attempts to isolate the stable N-acetyl Lys conjugate and characterize its structure via NMR have been unsuccessful.
Scheme 2.
Reaction of DOPAL may involve amine modification (Schiff base) followed by catechol oxidation and subsequent rearrangement to a more stable indole-structure
Although DA and DOPAC require catechol auto-oxidation for protein reactivity, DOPAL does not. However, the ability of DOPAL’s catechol to oxidize to a quinone greatly enhances its reactivity and toxicity, and DOPAL is highly reactive at physiologic pH. Therefore, maintaining adequate levels of cellular antioxidants is vital to preserving function under conditions of elevated DOPAL levels. An antioxidant-rich, i.e. reducing environment could prevent oxidation and subsequent protein modification. Inherently low levels of antioxidants generally leave the central nervous system susceptible to oxidative damage.3 Specifically, GSH levels tend to be depleted in AD and PD.44, 45 Such an environment would yield proteins particularly susceptible to modification by DOPAL.
Brains of PD and AD patients also show increased levels of the lipid peroxidation products 4HNE and MDA.2, 44 These reactive intermediates inhibit the enzymes responsible for DOPAL metabolism, which could lead to a build up of DOPAL in effected cells.4, 5, 25 In fact, an elevated ratio of levels of DOPAL:DOPAC has been observed in post mortem PD brains.8 Furthermore, protein aggregation in the form of Lewy Bodies is a hallmark of the disease.46 Lewy bodies are also prominent in Alzheimer’s Disease and Diffuse Lewy Body Disease, though their role is unclear.47, 48
The reactivity of DOPAL with proteins represents an unusual and distinct mechanism for a biogenic aldehyde as it appears to involve Schiff base chemistry but requires catechol oxidation, whether it be concerted or subsequent to Schiff base formation. The effects of antioxidant treatment on cellular toxicity and dysfunction also need to be elucidated. Clarifying the role of DOPAL in neurodegenerative disease is important for identifying appropriate PD interventions, such as ALDH activation.
Acknowledgments
Funding information
Supported by NIH grants R01 ES015507, R01 CA169046, P42 ES013661. The University of Iowa ESR Facility provided invaluable support via NIH P30 CA086862.
We gratefully acknowledge the technical assistance of Brett A. Wagner with EPR spectroscopy.
Abbreviations list
- ALDH
aldehyde dehydrogenase
- AscH−
ascorbate monoanion
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- EPR
electron paramagnetic resonance
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSH
glutathione
- MAO
monoamine oxidase
- NAC
N-acetyl cysteine
- NaCNBH3
sodium cyanoborohydride
- NAL
N-acetyl lysine
- NBT
nitroblue tetrazolium
- PD
Parkinson’s disease
- VMAT2
vesicular monoamine transporter
Footnotes
Supporting Information
Not Applicable
References
- 1.O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 2.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol. Rev. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 3.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 4.Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA. Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007;28:76–82. doi: 10.1016/j.neuro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J. Neurosci. Res. 2000;60:552–558. doi: 10.1002/(SICI)1097-4547(20000515)60:4<552::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson's disease. J. Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein DS. Biomarkers, mechanisms, and potential prevention of catecholamine neuron loss in Parkinson disease. Adv. Pharmacol. 2013;68:235–272. doi: 10.1016/B978-0-12-411512-5.00012-9. [DOI] [PubMed] [Google Scholar]
- 10.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson's disease pathogenesis. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- 11.Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur. J. Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson's disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. MAO-B elevation in mouse brain astrocytes results in Parkinson's pathology. PLoS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoud ST, Vecchio LM, Bergeron Y, Hossain MM, Nguyen LT, Bermejo MK, Kile B, Sotnikova TD, Siesser WB, Gainetdinov RR, Wightman RM, Caron MG, Richardson JR, Miller GW, Ramsey AJ, Cyr M, Salahpour A. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol. Dis. 2015;74:66–75. doi: 10.1016/j.nbd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O'Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. U S A. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82:419–426. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungar F, Tabakoff B, Alivisatos SG. Inhibition of binding of aldehydes of biogenic amines in tissues. Biochem. Pharmacol. 1973;22:1905–1913. doi: 10.1016/0006-2952(73)90050-6. [DOI] [PubMed] [Google Scholar]
- 20.Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 22.Mexas LM, Florang VR, Doorn JA. Inhibition and covalent modification of tyrosine hydroxylase by 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite. Neurotoxicology. 2011;32:471–477. doi: 10.1016/j.neuro.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinsmaa Y, Florang VR, Rees JN, Mexas LM, Eckert LL, Allen EM, Anderson DG, Doorn JA. Dopamine-derived biological reactive intermediates and protein modifications: Implications for Parkinson's disease. Chem. Biol. Interact. 2011;192:118–121. doi: 10.1016/j.cbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem. Res. Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- 26.Vermeer LM, Florang VR, Doorn JA. Catechol and aldehyde moieties of 3,4-dihydroxyphenylacetaldehyde contribute to tyrosine hydroxylase inhibition and neurotoxicity. Brain Res. 2012;1474:100–109. doi: 10.1016/j.brainres.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall GB, Chen J, Mebi CA, Okumura N, Swenson MT, Ossowski SE, Zakai UI, Nichol GS, Lichtenberger DL, Evans DH, Glass RS. Redox Chemistry of Noninnocent Quinones Annulated to 2Fe2S Cores. Organometallics. 2013;32:6605–6612. [Google Scholar]
- 28.Hastings TG, Lewis DA, Zigmond MJ. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson's disease. Adv. Exp. Med. Biol. 1996;387:97–106. doi: 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- 29.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 30.Rosengren E, Linder-Eliasson E, Carlsson A. Detection of 5-Scysteinyldopamine in human brain. J. Neural. Transm. 1985;63:247–253. doi: 10.1007/BF01252029. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson GE, Tottmar O. Biogenic aldehydes in brain: on their preparation and reactions with rat brain tissue. J. Neurochem. 1987;48:1566–1572. doi: 10.1111/j.1471-4159.1987.tb05702.x. [DOI] [PubMed] [Google Scholar]
- 32.Fellman JH. The rearrangement of epinephrine. Nature. 1958;182:311–312. doi: 10.1038/182311a0. [DOI] [PubMed] [Google Scholar]
- 33.Grande HJ, van der Ploeg KR. Tricine radicals as formed in the presence of peroxide producing enzymes. FEBS. Lett. 1978;95:352–356. doi: 10.1016/0014-5793(78)81028-x. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima H, Amano W, Kubo T, Fukuhara A, Ihara H, Azuma YT, Tajima H, Inui T, Sawa A, Takeuchi T. Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. J. Biol. Chem. 2009;284:34331–34341. doi: 10.1074/jbc.M109.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. U S A. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanghani PC, Stone CL, Ray BD, Pindel EV, Hurley TD, Bosron WF. Kinetic mechanism of human glutathione-dependent formaldehyde dehydrogenase. Biochemistry. 2000;39:10720–10729. doi: 10.1021/bi9929711. [DOI] [PubMed] [Google Scholar]
- 37.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 38.Ates B, Abraham L, Ercal N. Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC) Free Radic. Res. 2008;42:372–377. doi: 10.1080/10715760801998638. [DOI] [PubMed] [Google Scholar]
- 39.Gotoh N, Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochim. Biophys. Acta. 1992;1115:201–207. doi: 10.1016/0304-4165(92)90054-x. [DOI] [PubMed] [Google Scholar]
- 40.Alivisatos SG, Ungar F, Callaghan OH, Levitt LP, Tabakoff B. Inhibition of the formation of tetrahydroisoquinoline alkaloids in brain homogenates. Can. J. Biochem. 1973;51:28–38. doi: 10.1139/o73-005. [DOI] [PubMed] [Google Scholar]
- 41.Carey FA. Organic Chemistry. 6. New York: McGraw-Hill; 2006. [Google Scholar]
- 42.Song Y, Buettner GR. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helander A, Tottmar O. Reactions of biogenic aldehydes with hemoglobin. Alcohol. 1989;6:71–75. doi: 10.1016/0741-8329(89)90076-1. [DOI] [PubMed] [Google Scholar]
- 44.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 45.Perry TL, Godin DV, Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 46.Forno LS. Neuropathology of Parkinson's disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer's disease. J. Mol. Neurosci. 2001;17:225–232. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- 48.Weisman D, McKeith I. Dementia with Lewy bodies. Semin. Neurol. 2007;27:42–47. doi: 10.1055/s-2006-956754. [DOI] [PubMed] [Google Scholar]