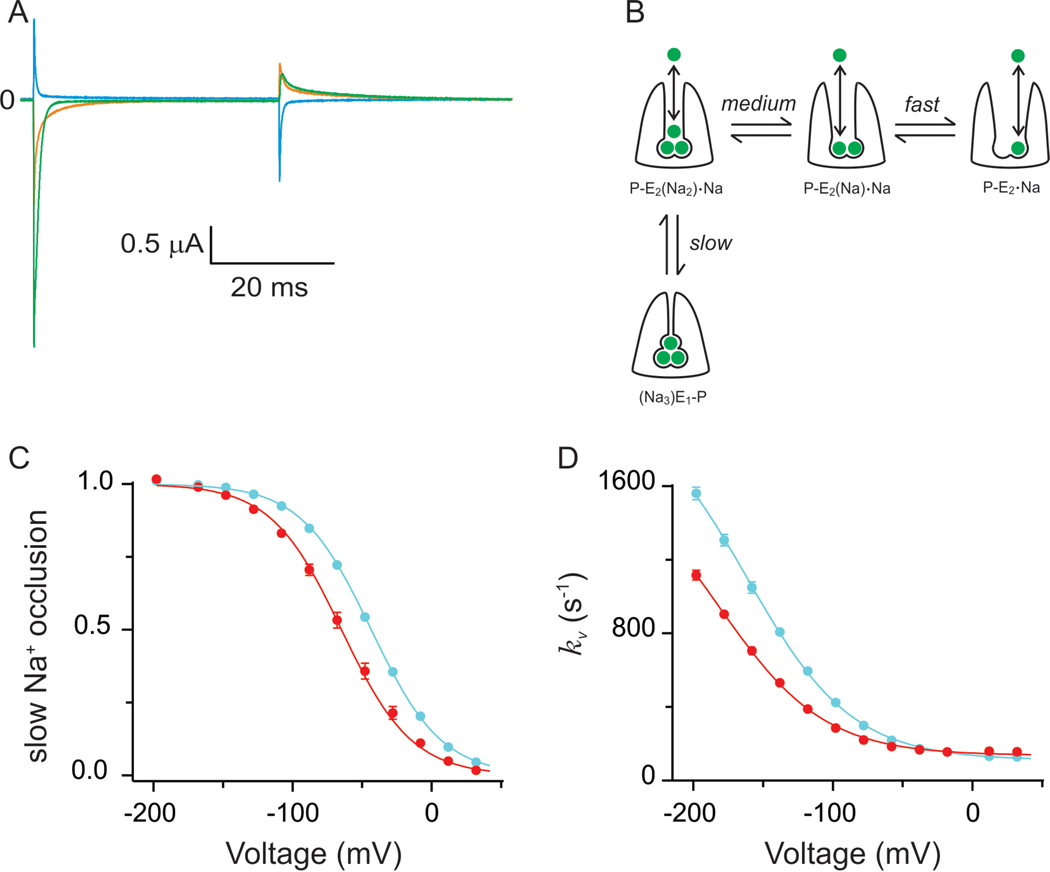
Figure 4.
RNA editing of squid Na+/K+-ATPase: functional consequences. (A) Transient currents mediated by the Na+/K+-ATPase trapped in states linked to release of external Na+. Green, orange and cyan current traces represent pump currents in response to fast voltage steps from 0mV to −198, −78 and +42mV, respectively. It is noticeable the multi component nature of these pump-mediated transient currents. (B) Cartoon representing the kinetic scheme that best describes the transient currents shown in (A). In this model, each of the Na+ binding/release and occlusion/deocclusion transitions are kinetically distinct. The slow component symbolizes the deep occlusion transition, which is the rate limiting step of the transient pump currents. (C) Voltage distribution of the deep occlusion transition. Cyan and red circles represent deep occlusion of unedited (I877) and edited (V877) pumps. Solid lines represent Boltzmann fits. RNA editing shifted the distribution towards more negative potentials which translate in faster pump velocities at potentials near the resting state of a neuron. (D) Voltage dependence of the relaxation rate of the deep occlusion transition. Same colour scheme as C. Solid lines correspond to ‘access channel model’ fits. Edited pumps reduced their apparent affinity for Na+ binding to ∼13M from a value of ∼7M in unedited pumps.