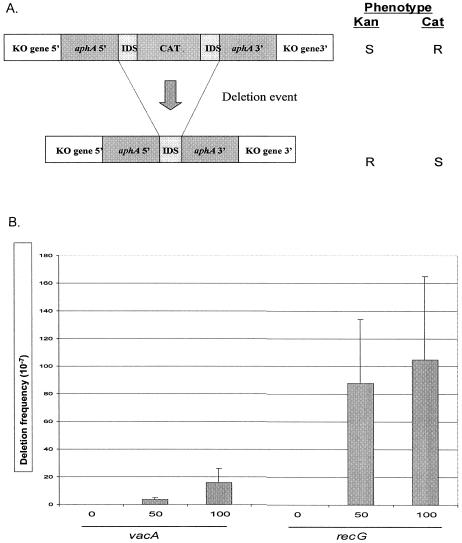
FIG. 3.
Intragenomic recombination in H. pylori wild-type and mutant strains. A. Schematic of constructs used to assess deletion frequency in H. pylori. H. pylori mutants were created by inserting a deletion cassette construct with either 0-bp (control), 50-bp, or 100-bp flanking identical repeat segments (IDS) in recA, vacA, and recG (indicated as knockout [KO] gene 5′ and KO gene 3′). One copy of the IDS is part of the 5′ region of aphA, and the other has been ligated to the chloramphenicol (CAT) cassette. Insertion of the complete cassette into a host H. pylori strain confers resistance to chloramphenicol. The chloramphenicol cassette can subsequently be deleted by recombination between the two flanking IDS DNA repeats, resulting in resistance (R) to kanamycin and susceptibility (S) to chloramphenicol. B. Deletion frequencies. The values are the means ± standard deviations (error bars) from four to six replicate experiments. As expected, H. pylori strains with the cassette in vacA (control) showed progressively higher deletion frequencies with increasing size of the IDS. Strains with the deletion cassette in recG show significantly (25- and 7-fold) higher intragenomic recombination frequencies between flanking DNA repeats of 50 and 100 bp, respectively (P values of <0.05) compared to the control mutants with comparable cassettes in vacA.