Abstract
Secreted phospholipase A2s (sPLA2s) regulate eicosanoid formation and have been implicated in asthma. Although sPLA2s function as enzymes, some of the sPLA2s bind with high affinity to a C-type lectin receptor, called PLA2R1, which has functions in both cellular signaling and clearance of sPLA2s. We sought to examine the expression of PLA2R1 in the airway epithelium of human subjects with asthma and the function of the murine Pla2r1 gene in a model of asthma. Expression of PLA2R1 in epithelial brushings was assessed in two distinct cohorts of children with asthma by microarray and quantitative PCR, and immunostaining for PLA2R1 was conducted on endobronchial tissue and epithelial brushings from adults with asthma. C57BL/129 mice deficient in Pla2r1 (Pla2r1−/−) were characterized in an ovalbumin (OVA) model of allergic asthma. PLA2R1 was differentially overexpressed in epithelial brushings of children with atopic asthma in both cohorts. Immunostaining for PLA2R1 in endobronchial tissue localized to submucosal glandular epithelium and columnar epithelial cells. After OVA sensitization and challenge, Pla2r1−/− mice had increased airway hyperresponsiveness, as well as an increase in cellular trafficking of eosinophils to the peribronchial space and bronchoalveolar lavage fluid, and an increase in airway permeability. In addition, Pla2r1−/− mice had more dendritic cells in the lung, higher levels of OVA-specific IgG, and increased production of both type-1 and type-2 cytokines by lung leukocytes. PLA2R1 is increased in the airway epithelium in asthma, and serves as a regulator of airway hyperresponsiveness, airway permeability, antigen sensitization, and airway inflammation.
Keywords: asthma, allergy, phospholipase A2, phospholipase A2 receptor 1, C-type lectin
Clinical Relevance
The present study identifies a novel C-type lectin receptor, called phospholipase A2 receptor 1 (PLA2R1), that is overexpressed in the airway epithelium of children with asthma and localizes to airway epithelial cells and submucosal glandular epithelium in adults with asthma. Functional evaluation of the murine homolog, Pla2r1, in a model of allergic inflammation found that the receptor serves as a regulator of allergic inflammation, airway permeability, and airway hyperresponsiveness.
Eicosanoids, such as cysteinyl leukotrienes, are elevated in the airways of patients with asthma (1, 2) and contribute to the development of allergic inflammation in murine models (3–5). The rate-limiting step in eicosanoid formation is phospholipase A2 (PLA2)–mediated release of arachidonate from the sn-2 position of membrane phospholipids. A total of 10 mammalian secreted PLA2s (sPLA2s) have been identified that may serve as key regulators of eicosanoid synthesis (6–8), often acting in concert with the well described group IVA cytosolic PLA2 (i.e., cPLA2α) (9, 10).
We have identified sPLA2 group 2A (sPLA2-IIA), 5 (sPLA2-V), and 10 (sPLA2-X) in the airways of patients with asthma (11, 12). It is clear that the majority of the sPLA2 activity in the airways of humans is mediated by sPLA2-IIA and sPLA2-X (12), and that the amount of sPLA2-X protein is increased in asthma (13). In murine models of asthma, sPLA2-X plays a key role in the ovalbumin (OVA) with aluminum potassium sulfate (alum) adjuvant model of allergic inflammation (14, 15), and sPLA2-V is involved in the OVA model (16) as well as house dust mite–mediated allergic inflammation (17, 18). Although the sPLA2s act as enzymes with varying affinity for the release of free fatty acids from mammalian cells (19, 20), a receptor has also been identified that binds to sPLA2s.
PLA2 receptor 1 (PLA2R1) is a 180-kD type 1 or integral transmembrane receptor with a large extracellular domain and a short cytoplasmic domain (21). The receptor has varying affinities for the different sPLA2s, and may also act as a pleotropic receptor by binding non-sPLA2 ligands (22). In fact, the receptor belongs to the superfamily of C-type lectins, and is a paralog of the macrophage mannose receptor and other receptors within this superfamily (23). The protein is transcribed as either a transmembrane form or a shortened secreted form (24), suggesting functions in both cellular signaling (25–27) as well as clearance of sPLA2s (28–30). The soluble form of the receptor can also be generated by proteolytic cleavage (27). A prior investigation of the function of murine Pla2r1 in a low-dose OVA model found that the clearance of sPLA2 group IB (sPLA2-IB) from the lung was reduced in Pla2r1-deficient (Pla2r1−/−) mice, and that airway inflammation was increased, but airway hyperresponsiveness (AHR) and quantitative lung morphometry were not reported (31).
In a genome-wide expression study of epithelial cells, we identified increased expression of the human PLA2R1 gene in the epithelium of children with allergic asthma. We then characterized the expression of PLA2R1 in a second cohort of children with and without asthma, and characterized the location of PLA2R1 immunostaining in endobronchial airway biopsies of adults with asthma. We examined the effects of the murine Pla2r1 gene deletion in an OVA model with an exogenous adjuvant and assessed the effects on AHR, airway morphometry, trafficking of leukocytes to the airways and lung, airway permeability, production of key cytokines by lung leukocytes, and allergen-specific IgE and IgG.
Materials and Methods
Epithelial Microarray and Quantitative PCR Analysis
We initially conducted an analysis of PLA2-related epithelial gene expression using microarrays that we performed on epithelial brushings from children with and without mild atopic asthma (AA) (32). In this cohort, children were recruited before elective surgery and characterized based on the presence or absence of a physician diagnosis of asthma and a radioallergosorbent result to a panel of common allergens. Children with asthma had not taken inhaled or oral corticosteroids for at least 1 month before surgery. Epithelial cells were collected by nonbronchoscopic cytology brushings collected at the time of surgery. For the microarray analysis, RNA from epithelial brushings of seven children with AA and nine healthy, nonatopic (HNA) control subjects was hybridized to the Affymetrix Human Genome U133A Array (Affymetrix, Santa Clara, CA). Differential gene expression between the groups was assessed after normalization by GC robust multiarray algorithm based on the adjusted P value and false discovery rate.
To further corroborate these results, we used quantitative PCR to assess the gene expression of PLA2R1 from epithelial brushings from a distinct cohort of nine children with AA and nine HNA children as control subjects. TaqMan primer probe sets (Thermo Fisher Scientific, Waltham MA) were used to assess the expression of PLA2R1 (Hs00234853_m1) relative to the expression of PPIA (cyclophilin A; Hs99999904_ml) as an endogenous control. The internal review board of the University of Western Australia (Perth, WA, Australia) approved the pediatric airway epithelial studies.
Immunohistochemistry of Endobronchial Biopsies
To localize the expression of PLA2R1 protein in the airways, we conducted immunostaining using endobronchial biopsies and epithelial brushings from a cohort of subjects with asthma who had undergone research bronchoscopy (13). Endobronchial biopsies were obtained from second- to fifth-generation carina of the right lower and middle lobes using 1.8-mm forceps. The biopsies were fixed in methyl Carnoy’s solution before embedding in paraffin. Immunostaining for PLA2R1 was localized using a rabbit polyclonal anti-PLA2R1 antibody that was generated in the laboratory of G.L. (33). The biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was visualized with 3,3′-diaminobenzidine with nickel chloride enhancement. No immunostaining was seen with a rabbit polyclonal isotype control antibody that was used as a negative control (34). The internal review board of the University of Washington (Seattle, WA) approved the research bronchoscopy studies to acquire these samples.
OVA with Exogenous Adjuvant Model of Asthma
C57BL/129 Pla2r1−/− mice and wild-type (WT) Pla2r1+/+ controls received intraperitoneal sensitization with 100 μg of endotoxin-free OVA (vac-pova; InvivoGen, San Diego, CA) complexed with alum (Thermo Scientific, Waltham, MA) on Days 0 and 14. The mice received an intranasal dose of 100 μg OVA on Day 14, and 50 μg OVA on Days 26–28. Control groups received 0.2 ml normal saline with alum intraperitoneally on Days 0 and 14 and saline without intranasal alum on Days 14 and 26–28. On Day 29, mice were intubated and ventilated using a flexiVent ventilator (SCIREQ Inc., Montreal, PQ, Canada), and lung function assessed after increasing concentrations of inhaled methacholine. Dynamic lung resistance was calculated using the single-frequency forced oscillation technique based on a 1-second broadband perturbation (35). The University of Washington Institutional Animal Care and Use Committee approved the murine studies.
Assessment of Allergen-Induced Inflammation
Bronchoalveolar lavage (BAL) fluid was collected and the cell differential was enumerated by flow cytometry. The left lung was digested in collagenase D and DNase, and, after magnet-assisted positive selection, CD45-positive leukocytes were further enumerated by flow cytometry (Figure E1). Suspensions of lung leukocytes were placed in 96-well cell culture plates for 24 hours either with/or without antigen stimulation with OVA (100 μg/ml). Cell culture supernatants were assayed for the levels of cytokines by murine V-plex assay (Meso Scale Diagnostics, Rockville, MD). Mouse plasma samples were assayed for OVA-specific IgE and IgG1 by ELISA (Cayman, Ann Arbor, MI). Detailed methods for the murine model are presented in the online supplement.
Mouse Lung Histopathology
The right lung was inflated and fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections for histopathology analyses. The sections were immunostained for eosinophils using a rat anti–major basic protein (MBP) antibody that was generously provided by J. J. Lee (Mayo Clinic, Phoenix, AZ) (36, 37). To quantify the density of eosinophils in the lung, deconvolution analysis was conducted using the Visiopharm system (Visiopharm, Hoersholm, Denmark) to quantify total MBP immunostaining in the lung. We used segmentation to determine the number of eosinophils in the lung. To quantify eosinophils surrounding the airways, we outlined the submucosal space surrounding the airways as the region of interest to define the peribronchial area. Within this peribronchial area, we quantified the total MBP immunostaining using deconvolution, and the number of eosinophils using segmentation. To quantify the density of mucus-producing cells, we quantified the amount of Alcian blue/periodic acid–Schiff (PAS) staining relative to the area of the epithelium and relative to the length of the basal lamina.
Statistical Analysis
Details of the statistical analysis of the microarray were reported previously (32). We assessed the relationship between the expressions of the different PLA2-related genes using linear regression analyses. Comparison of epithelial gene expression based on PCR was assessed with an unpaired t test. Alterations in AHR to methacholine were assessed with a two-way ANOVA with contrasts between methacholine dose and genotype. Post hoc tests of each dose step were assessed after correction for multiple comparisons with Bonferroni’s test. Differences in features of inflammation, including the differential cells counts, cytokine production by antigen-stimulated leukocytes, and quantification of immunostaining, were assessed with a two-way ANOVA with contrasts of allergen treatment and genotype. Post hoc comparisons for inflammatory parameters were made with Fisher’s least significant difference.
Results
Among the PLA2-Related Genes, PLA2R1 Is the Most Differentially Expressed in Epithelial Brushings of Children with and without Asthma
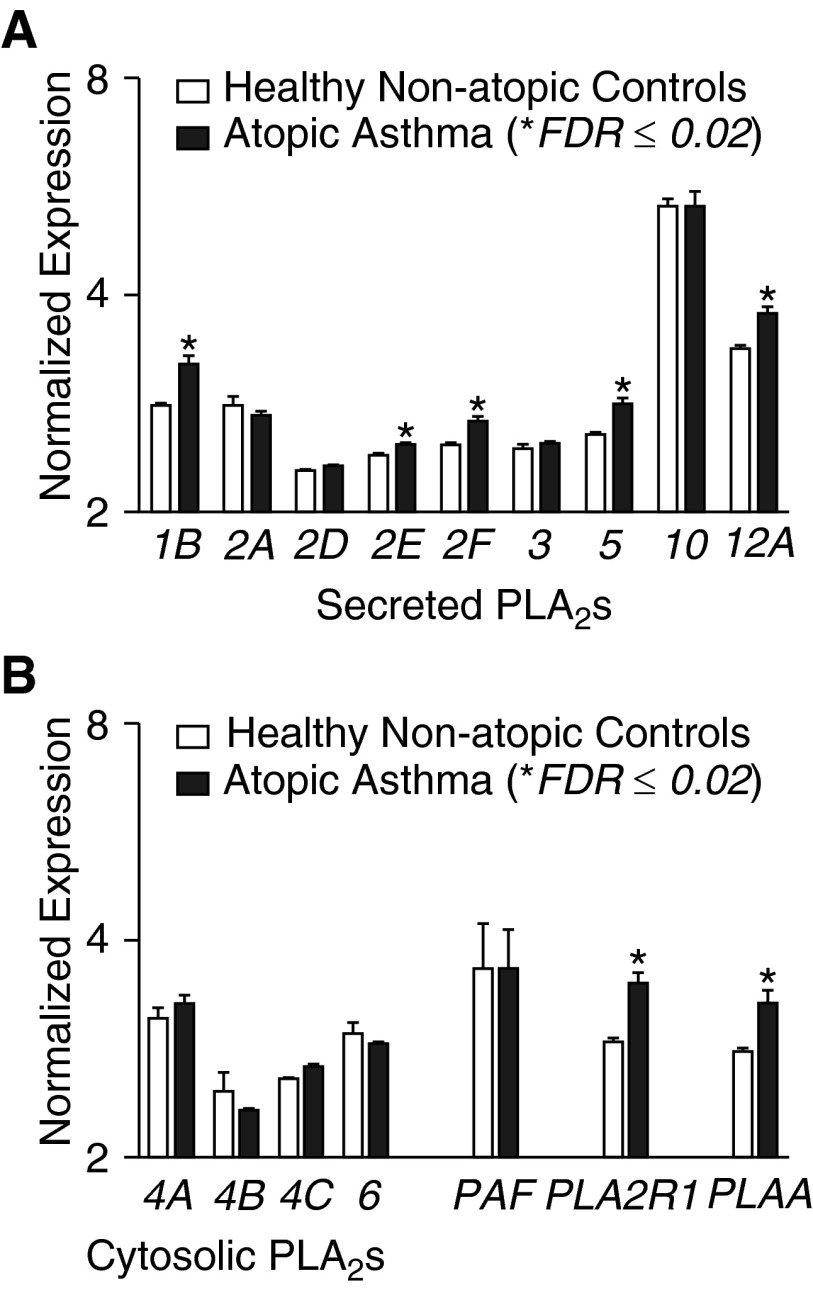
We assessed differences in the expression of PLA2s and PLA2-related genes in the epithelium in asthma using oligonucleotide array data that we collected on a cohort of children with AA relative to HNA control subjects (Figure 1). The results of this focused analysis reveal that secreted PLA2 groups 1B, 2E, 2F, 5, and 12A were each differentially expressed in asthma at a false discovery rate 0.02 or less, but the magnitude of these differences was modest (see additional details in Table E1). We found no differences in the expression of any of the cytosolic PLA2s, including the calcium-dependent groups 4A, 4B, and 4C or the calcium-independent group 6. Similarly, there was no alteration in the expression of sPLA2 group 7, also known as platelet-activating factor acetylhydrolase. The genes with the greatest differential gene expression in this group based on the P value and fold change were PLA2R1 and PLA2-activating protein. We found that there were strong positive correlations between the expression of PLA2R1 and the expression levels of PLA2G1B, the group II sPLA2s, PLA2G5, PLA2G12A, and PLA2-activating protein, whereas PLA2G10 had a weak inverse correlation with the expression of PLA2R1 (Figure E2 and Table E2).
Figure 1.
Differential expression of phospholipase A2s (PLA2s) and related genes in the airway epithelium of asthma. Microarrays assessed the expression of PLA2-related genes from epithelial brushings of children with atopic asthma (AA) relative to healthy, nonatopic (HNA) control subjects. (A) Expression of secreted PLA2 groups 1B, 2E, 2F, 5, and 12A were differentially expressed at a false discovery rate (FDR) of 0.02 or less. (B) Cytosolic PLA2s and platelet activating factor acetylhydrolase (PAF) were not differentially expressed, whereas PLA2R1 and PLA2-activating protein (PLAA) were differentially expressed (*FDR ≤ 0.02). Data are represented as means ± SEM.
Differential Expression of PLA2R1 Was Confirmed in a Distinct Cohort and Localized to Glandular and Nonciliated Epithelium
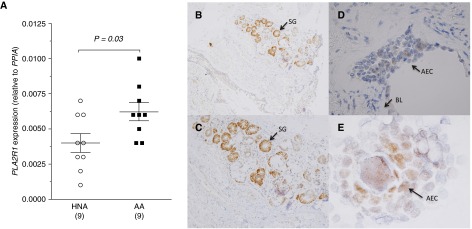
To further corroborate the differences in gene expression of PLA2R1 in the epithelium, we assayed the gene expression by quantitative PCR in a new cohort of children with and without asthma. The epithelial gene expression of PLA2R1 relative to PPIA (cyclophilin A) was increased in children with AA relative to HNA control subjects by approximately 1.5 fold (Figure 2A).
Figure 2.
Localization of PLA2R1 in endobronchial airway tissue. (A) Differential expression of PLA2R1 in epithelial brushings was confirmed in a distinct cohort of children with and without asthma. Data are represented as means ± SEM. (B–D) Immunostaining for PLA2R1 in endobronchial tissue shows prominent immunostaining in submucosal glandular epithelium shown at (B) 10× brightfield and (C) 40× brightfield. (D) Immunostaining is also evident in columnar airway epithelial cells at 60× oil. (E) Epithelial cytospin preparations at 100× oil further demonstrate immunostaining for PLA2R1 in columnar epithelial cells. Arrows indicate submucosal glands (SG, panels B and C), basal lamina (BL, panel D), and airway epithelial cell (AEC, panel E).
To further define the location of epithelial immunostaining in asthma, we performed immunostaining on endobronchial biopsies and epithelial brushings from a repository of samples from adults with asthma (13). On a low-power brightfield image (10×), immunostaining is readily apparent in submucosal glandular epithelium, with fainter immunostaining in the airway epithelium (Figure 2B). At higher power (40× brightfield), immunostaining is readily apparent in the submucosal glandular epithelium (Figure 2C). At higher power (60× oil) focused on the airway epithelium, staining for PLA2R1 is identified in the columnar epithelium (Figure 2D). Immunostaining in columnar epithelial cells was also identified on cytospin preparations of epithelial cells from bronchial brushings (100× oil, Figure 2E).
Deletion of Pla2r1 Increases AHR and Eosinophilic Airway Inflammation, and Alters the Levels of sPLA2-X in a Mouse Asthma Model
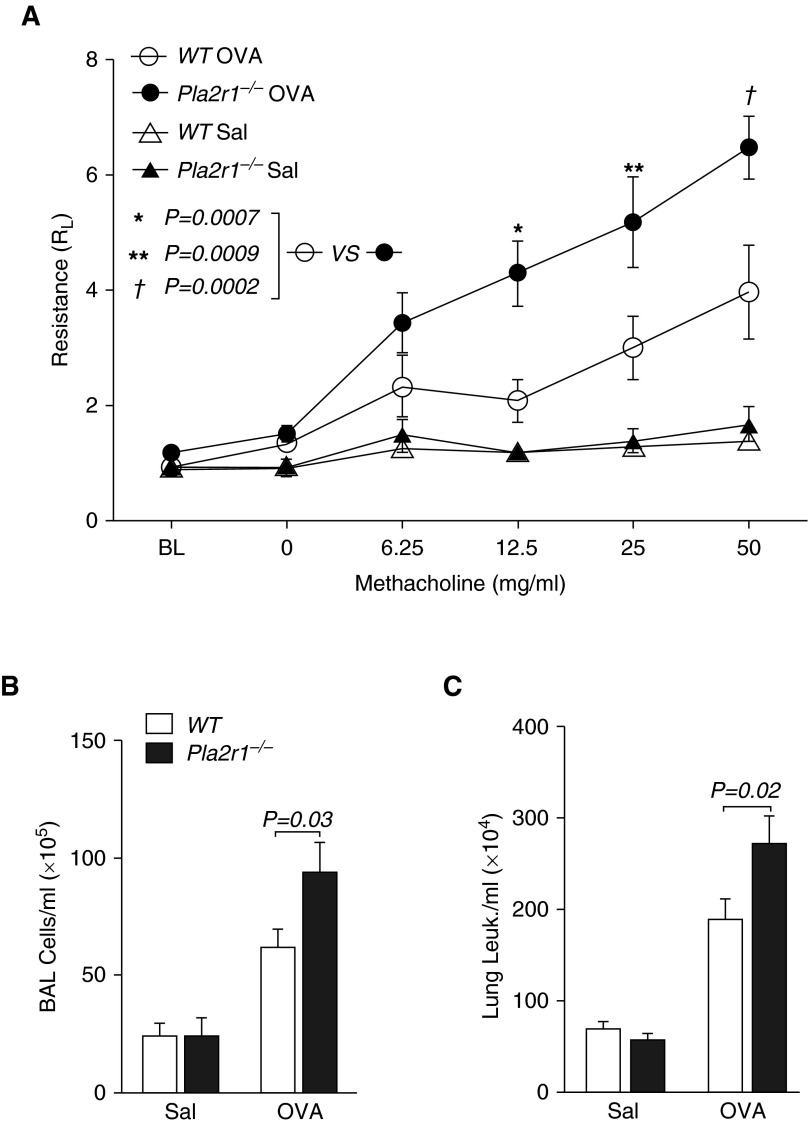
To examine the function of Pla2r1, we examined the effects of a global deletion of the Pla2r1 gene in a murine model of sensitization with OVA and alum, followed by airway challenge with OVA. Prior work demonstrated that mice express Pla2r1 in the airway epithelium and airway smooth muscle (31). We found that Pla2r1−/− mice have increased AHR to methacholine after OVA sensitization and challenge (P = 0.0005 overall genotype effect two-way ANOVA; Figure 3A), and specifically at the final three methacholine dose steps (P = 0.0002–0.0009). After OVA sensitization and challenge, the concentration of cells in BAL fluid was greater in Pla2r1−/− mice relative to WT controls (P = 0.03, Figure 3B). The number of BAL CD45+ leukocytes was similarly increased in the Pla2r1−/− mice (P = 0.01, data not shown). We also examined the total concentration of CD45+ leukocytes in the lung after BAL and removal of circulating cells, and found that the concentration of leukocytes was increased in the lungs of the Pla2r1−/− mice after sensitization and challenge relative to WT controls (P = 0.02, Figure 3C). As we have previously identified a major role for sPLA2-X in the OVA model of allergic inflammation (14, 15), we measured the levels of sPLA2-X by Western blot in BAL fluid, and found that the amount of sPLA2-X in saline-treated mice was markedly increased in Pla2r1−/− mice relative to WT mice (P = 0.0006), but this difference was no longer apparent after OVA sensitization and challenge (Figure E3).
Figure 3.
Changes in airway mechanics and airway inflammation in Pla2r1-deficient (Pla2r1−/−) mice. (A) Airway hyperresponsiveness (AHR) to increasing doses of inhaled methacholine revealed increased AHR in Pla2r1−/− mice relative to wild-type (WT) mice after ovalbumin (OVA) sensitization and challenge (P = 0.0005 overall genotype effect, two-way ANOVA). (B) Bronchoalveolar lavage (BAL) cells (C) and lung leukocytes (Leuk.) were increased in Pla2r1−/− mice relative to WT mice after OVA treatment. Data are represented as means ± SEM. *P = 0.0007, **P = 0.0009, †P = 0.0002. RL, lung resistance; Sal, saline.
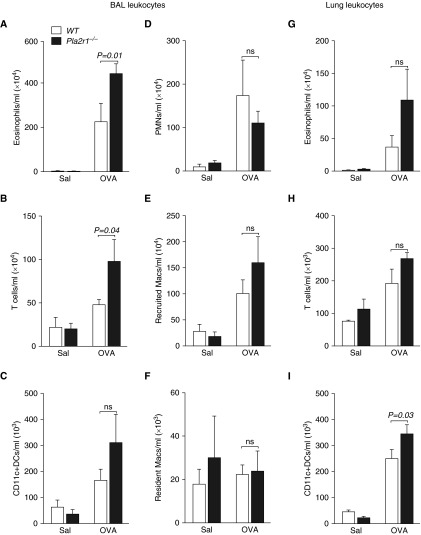
We assessed the concentration of leukocyte subsets in BAL fluid by flow cytometry and found that the concentration of eosinophils and T cells were increased in the Pla2r1−/− mice after sensitization and challenge, whereas there were no significant differences in the concentration of neutrophils, recruited macrophages, resident macrophages, or dendritic cells (DCs) (Figures 4A–4F). We similarly characterized leukocyte trafficking to the lung using flow cytometry of lung leukocytes isolated from lung tissue after both BAL and perfusion of the lung with saline to remove circulating leukocytes. We found that, although eosinophils, T cells, and CD11C+ DCs were increased after OVA treatment (Figures 4G–4I), only the number of DCs was significantly increased in the Pla2r1−/− mice. There were also no differences in the concentrations of neutrophils, recruited macrophages, or resident macrophages between the WT and Pla2r1−/− mice (data not shown).
Figure 4.
Alterations in leukocyte trafficking in the BAL fluid and lung tissue in Pla2r1−/− mice. Concentrations of (A) eosinophils and (B) T cells were increased in Pla2r1−/− mice in the BAL fluid after OVA, whereas there were no differences in the concentrations of (C) dendritic cells (DCs), (D) polymorphonuclear neutrophils (PMNs), (E) recruited macrophages (Macs), or (F) resident macrophages relative to WT mice exposed to OVA. Although the concentrations of (G) eosinophils and (H) T cells were unchanged, there was an increase in the concentration of (I) DCs in lung tissue from Pla2r1−/− mice exposed to OVA relative to WT controls. ns, not significant. Data are represented as means ± SEM.
Alterations in the Airway Wall and Increased Airway Permeability in Pla2r1−/− Mice
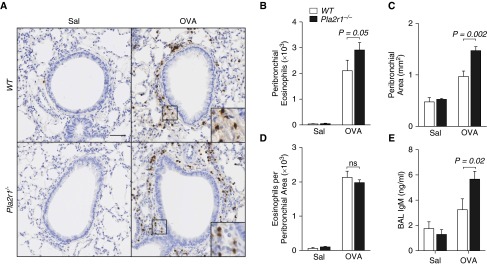
We quantified the number of eosinophils in the peribronchial space using immunohistochemistry for the eosinophil granule protein, MBP. Representative images are shown in Figure 5A. Segmentation analysis of the peribronchial space, using an unbiased automated system that counts MBP-positive cells, demonstrated that the total number of eosinophils surrounding the airways was increased in Pla2r1−/− mice relative to WT mice after OVA (Figure 5B). We also quantified the submucosal space surrounding the airways using image analysis; this analysis revealed that the peribronchial area was increased in the Pla2r1−/− mice relative to WT mice after OVA (Figure 5C). Because the area surrounding the airways was increased in the Pla2r1−/− mice, there was no difference between the WT and Pla2r1−/− mice for the number of eosinophils per area surrounding the airways (Figure 5D). Although we did not further characterize the composition of the space surrounding the airways, we examined the degree of airway permeability after methacholine challenge using the large protein IgM, and found that the level of IgM in the BAL fluid was markedly elevated in the Pla2r1−/− mice relative to WT mice (Figure 5E). These results suggest that peribronchial remodeling contributes to AHR through increased airway permeability.
Figure 5.
Deficiency of Pla2r1 alters peribronchial area, eosinophilic inflammation, and airway permeability. (A) Representative images of eosinophil major basic protein (MBP) staining of lung tissue from saline- and OVA-exposed WT and Pla2r1−/− mice (original magnification, 20×; scale bar: 50 μM). Insets show detail of focal MBP staining in OVA-treated WT mice, representing peribronchial eosinophils. (B) Total peribronchial eosinophils and (C) the peribronchial area were increased in Pla2r1−/− mice after OVA challenge; however, the number of eosinophils per peribronchial area was unchanged (D). (E) Airway permeability based on the transit of IgM to the airway after methacholine challenge was increased in Pla2r1−/− mice exposed to OVA. Data are represented as means ± SEM.
Through the use of image analysis, we also quantified the total percent area of MBP staining, representing the amount of MBP within eosinophils as opposed to counting discrete cells. The total amount of MBP staining was actually decreased in the Pla2r1−/− mice, suggesting that the amount of MBP within the eosinophils was decreased (Figure E4A), consistent with increased degranulation and release of the granule protein MBP in these mice. To further corroborate this finding, we examined the amount of MBP in BAL fluid by Western blot and found that there was a notable trend toward an increase in MBP in the BAL fluid of the Pla2r1−/− mice relative to WT mice (P = 0.08, Figure E4B). We also quantified the total number of eosinophils in the lung by segmentation analysis and found that total lung eosinophils was not altered in the Pla2r1−/− mice after OVA (Figure E4C), consistent with our results by flow cytometry of eosinophils. The amount of MBP in the lung eosinophils tended to be decreased in the lungs of Pla2r1−/− mice (P = 0.13, Figure E4D), consistent with increased eosinophil degranulation in the Pla2r1−/− mice. Overall, these results demonstrate that there are more eosinophils surrounding the airways in Pla2r1−/− mice in association with enlargement of the peribronchial space, increased airway permeability, and increased eosinophil degranulation. These findings provide important insights into the mechanism of elevated AHR in the Pla2r1−/− mice.
The Amount of Mucin in the Airway Epithelium after Methacholine Challenge Is Reduced in Pla2r1−/− Mice
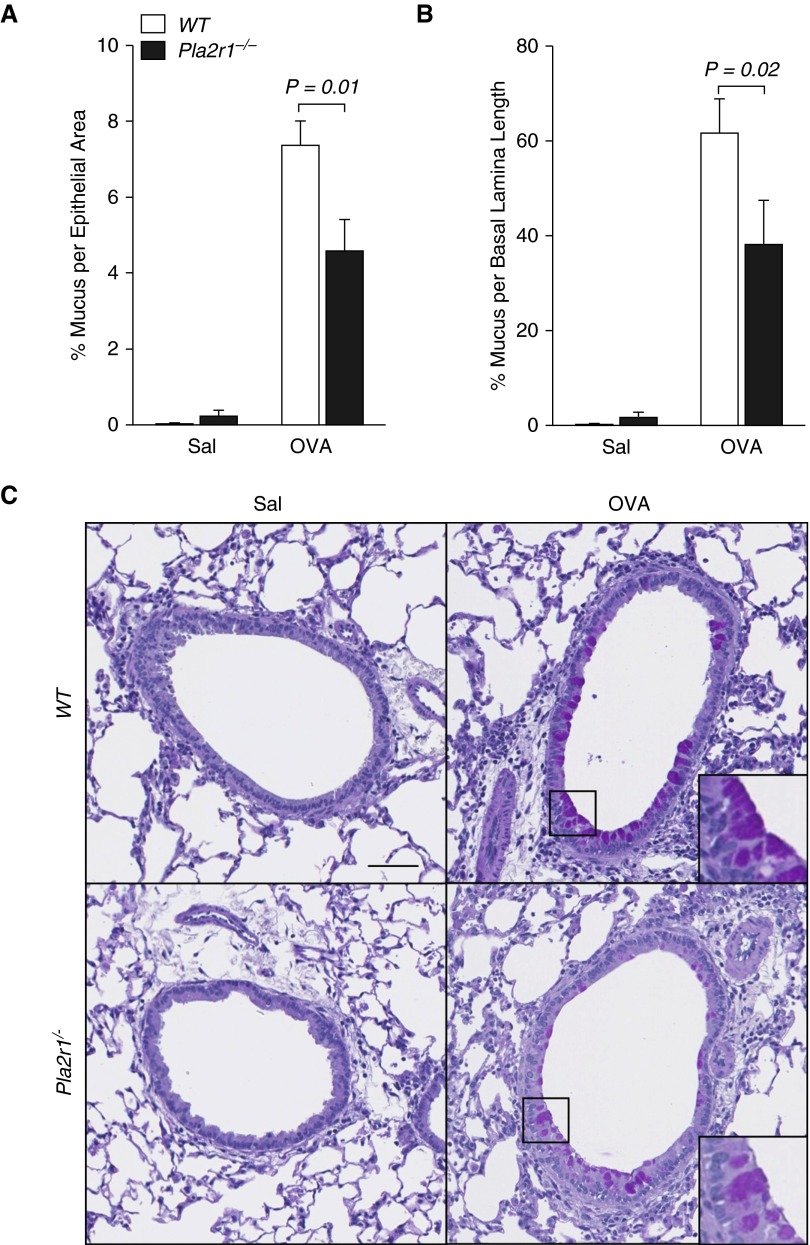
We quantified the amount of mucin staining in the epithelium using segmentation analysis, demonstrating that, with sensitization and challenge with OVA, there was a marked increase in the percent of mucin staining relative to the area of the epithelium, and the percent of mucin staining relative to the length of the basal lamina (P < 0.0001 for both measures). We conducted the PAS staining for mucin after BAL and after methacholine challenge. In this context, it was previously demonstrated, using quantitative morphometry, that there is an acute decrease in mucin content in the epithelium that represents the release of mucin from the epithelium (38). We found that there was a significant decrease in mucin staining relative to the epithelial area in the Pla2r1−/− mice relative to WT controls (P = 0.01, Figure 6A). Similarly, we found that the percentage of mucin staining relative to the length of the basal lamina was decreased in Pla2r1−/− mice relative to WT controls (P = 0.02, Figure 6B). These results are consistent with greater epithelial mucin release during methacholine challenge in the Pla2r1−/− mice. Representative PAS images are shown in Figure 6C.
Figure 6.
Mucin content in the airway epithelium is altered in Pla2r1−/− mice after methacholine challenge. Mucin content in lung tissue was determined by periodic acid–Schiff staining and quantified by segmentation analysis. The mucin content was increased after OVA, and (A) percentage of mucus per total epithelial area and (B) percentage of mucus per BL length were decreased in Pla2r1−/− after methacholine challenge, consistent with greater mucin release. (C) Representative images of mucin staining of lung tissue from WT and Pla2r1−/− mice exposed to OVA or saline (original magnification, 20×; scale bar: 50 μM). Insets show detail of mucin staining. Data are represented as means ± SEM.
Cytokine Production by Lung Leukocytes and Allergen-Specific IgG Are Increased in Pla2r1−/− Mice
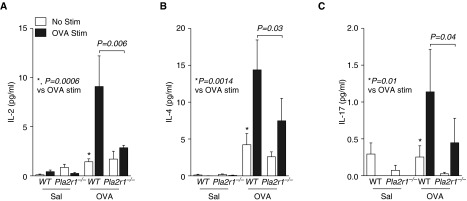
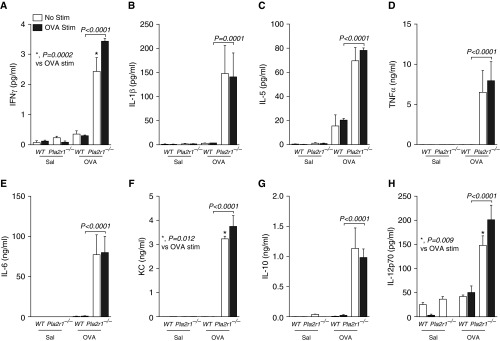
We isolated lung leukocytes after sensitization and challenge and stimulated these cells in vitro for 24 hours with saline control or OVA. We found that there was an OVA-induced increase in the generation of the cytokines, IL-2, IL-4, and IL-17 (Figure 7). The levels of IL-13 were below the level of detection in our assay. The OVA-induced generation of IL-2, IL-4, and IL-17 in OVA-sensitized mice was lower in the Pla2r1−/− mice relative to WT mice (Figure 7). In contrast, the generation of multiple cytokines that were not OVA induced in vitro was markedly higher in Pla2r1−/− mice relative to WT mice after sensitization and challenge with OVA (Figure 8). These cytokines included both type-1 and type-2 cytokines, including IFN-γ, IL-1β, IL-5, IL-6, IL-10, IL-12p70, chemokine (C-X-X motif) ligand 1 (CXCL1)/keratinocyte-derived chemokine (KC), and TNF-α.
Figure 7.
Differential antigen-dependent production of proinflammatory cytokines in Pla2r1−/− mice. Isolated lung leukocytes were cultured in the presence of OVA or saline for 24 hours and cell culture supernatant was assessed for cytokine release. There was an OVA-induced production of the cytokines, IL-2, IL-4, and IL-17, in WT mice exposed to OVA. The production of (A) IL-2, (B) IL-4, and (C) IL-17 were significantly decreased in OVA-restimulated cells isolated from Pla2r1−/− mice relative to OVA-restimulated cells from WT mice. (A) *P = 0.0006 versus OVA stimulated (Stim); (B) *P = 0.0014 versus OVA stimulated; (C) *P = 0.01 versus OVA stimulated.
Figure 8.
Antigen-independent production of proinflammatory cytokines is augmented in Pla2r1−/− mice. Isolated lung leukocytes were cultured in the presence of OVA or saline for 24 hours and cell culture supernatant was assessed for cytokine release. The levels of cytokines (A) IFN-γ, (B) IL-1β, (C) IL-5, (D) TNF-α, (E) IL-6, (F) chemokine (C-X-X motif) ligand 1 (CXCL1)/keratinocyte-derived chemokine (KC), (G) IL-10, and (H) IL-12p70 were significantly elevated in Pla2r1−/− mice relative to WT mice, although there was no evidence of an ex vivo increase in the production of these cytokines by OVA treatment. (A) *P = 0.0002 versus OVA stimulated; (F) *P = 0.012 versus OVA stimulated; (H) *P = 0.009 versus OVA stimulated. Data are represented as means ± SEM.
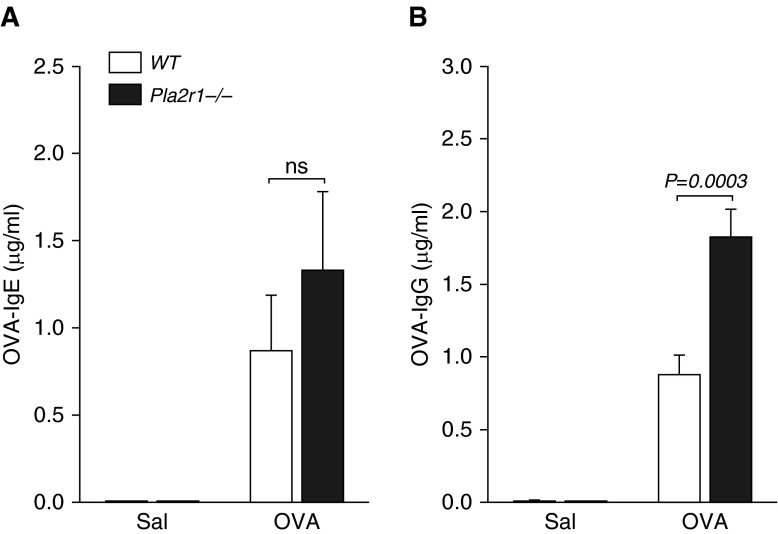
We found a significant increase in OVA-specific IgE in OVA-treated mice in both genotypes, and, although the OVA-specific IgE tended to be higher in the OVA-treated Pla2r1−/− mice, it did not reach statistical significance (P = 0.34, Figure 9A). There was a marked increase in OVA-specific IgG1 in OVA-treated mice (P < 0.01), and a significant increase in OVA-specific IgG1 in Pla2r1−/− mice relative to WT mice after treatment with OVA (P = 0.0003, Figure 9B).
Figure 9.
Effects of Pla2r1 deficiency on allergen-specific Igs. The levels of (A) OVA-specific IgE were not significantly elevated in Pla2r1−/− mice relative to WT controls; however, the levels of OVA-specific IgG were increased in Pla2r1−/− mice after OVA (B). Data are represented as means ± SEM.
Discussion
We identified human PLA2R1 as an epithelial gene that is overexpressed in asthma. Within the airways of patients with asthma, the receptor is abundantly expressed in the submucosal glands and in columnar epithelial cells. In a model of asthma, absence of Pla2r1 increased AHR to methacholine after sensitization and challenge, and increased eosinophil trafficking to the peribronchial space and BAL fluid. We identified an increase in the peribronchial area, a marked increase in airway permeability, increased eosinophil degranulation and greater mucin release that may contribute to this increase in AHR in the absence of Pla2r1. Our results further demonstrate that, in the absence of Pla2r1, there are marked alterations in leukocyte function notable for the increased production of a broad range of cytokines by lung leukocytes, including a notable increase in IL-5, as well as an increase the number of CD11c+ DCs in the lung and an increase OVA-specific IgG. These findings may be related to an increase in the level of sPLA2-X in the airways, which was markedly increased in Pla2r1−/− mice without allergic sensitization. These results suggest that Pla2r1 serves as a key regulator of allergic inflammation and AHR in mice, and that the overexpression of this receptor in the epithelium of human subjects with asthma could serve as a mechanism to limit airway inflammation.
Our study is the first to identify increased expression of PLA2R1 in the airway epithelium of subjects with asthma; however, it is known that several different high-affinity ligands for PLA2R1, including sPLA2-IIA, sPLA2-V, and sPLA2-X, are abundantly expressed in the skin (39, 40), and we have found that PLA2G10 is expressed at higher levels than either PLA2G2A or PLA2G5 in the airway epithelium (12). In the skin, the transgenic expression of sPLA2-IIA increases skin carcinogenesis (41), whereas Pla2r1−/− mice have increased sensitivity to carcinogen-induced skin tumorigenesis (42). As such, the marked immunostaining in glandular epithelium suggests that the release of the soluble form of the receptor could serve in the clearance of sPLA2s in the airways. The immunostaining for Pla2r1 in mice also indicates localization to the airway epithelium (31), implicating the epithelial expression of this receptor in the in vivo findings in this study; however, the immunostaining in the airway smooth muscle that was identified in mice is not readily apparent in our human endobronchial biopsy specimens.
Our results are consistent with a prior study by Tamaru and colleagues (31) that demonstrated an increase in BAL eosinophils in Pla2r1−/− mice; however, the effects on AHR, leukocyte trafficking to the lung, leukocyte function, and antigen-specific Igs were not previously assessed. Our results are critical to understanding the function of Pla2r1 because of the opposing functions of the receptor. The receptor is a C-type lectin receptor that is a paralog of the mannose receptor. Ligation of this receptor by high-affinity PLA2 ligands initiates cellular signaling events in cells such as macrophages (25–27). The soluble form of the receptor can be generated via alternatively transcribed forms (24), and also by proteolytic cleavage (27), although the specific protease has not been identified. The soluble form of PLA2R1 is involved in clearance of sPLA2s, particularly groups IB, IIA, IIE, IIF, and X, that have high affinity for this receptor (22), but enhanced clearance from the lung has only been demonstrated for sPLA2-IB (31), an enzyme with uncertain relevance to asthma pathogenesis. In addition, the transmembrane form of the receptor also mediates the internalization and removal of sPLA2s via the lysosomal pathway (28–30).
The widespread proinflammatory effect of the Pla2r1 deletion in the murine model suggests that the predominant effect was through the loss of the clearance mechanism mediated by this receptor. In fact, we found that the level of sPLA2-X in the BAL fluid was markedly elevated in Pla2r1−/− mice before sensitization and challenge. The one area where the loss of the receptor appeared to reduce leukocyte activation was on the OVA-induced production of IL-2, IL-4, and IL-17 by lung leukocytes, which were reduced in the Pla2r1−/− mice. Regardless, the overall effects of the Pla2r1 deletion were proinflammatory, including the widespread overproduction of cytokines by lung leukocytes, increased DCs in the lung, and higher levels of antigen-specific IgG1. As cytokines associated with activation of the innate immune system were increased in the Pla2r1−/− mice, it is notable that a recent study showed that bee venom PLA2, an enzyme that functions much like endogenous sPLA2s, was involved in generating Th2 type T cell responses in the periphery in a process that was dependent upon the generation of IL-33 and innate lymphoid cells (43).
A key finding in the present study was the marked increase in AHR in mice deficient in Pla2r1. The most plausible explanation for these findings is the increase in the peribronchial area, increased airway permeability, and increased degranulation of eosinophils in the airways noted in Pla2r1−/− mice. Studies using cationic proteins, such as poly-l-lysine, that mimic eosinophil cationic proteins and cause airway edema, substantially increase AHR to methacholine by increasing the thickness of the airway wall (35, 44). Another plausible explanation is a change in the permeability of endothelial cells in the Pla2r1−/− mice, as PLA2R1 is the major autoantigen in membranous nephropathy, a disease with increased permeability of highly specialized epithelial cells called podocytes. In the present study, we found that the large protein IgM transited into the BAL fluid in increased amounts in the Pla2r1−/− mice. In membranous nephropathy, the receptor itself may be involved in the development of the disease, as gene variants of the human PLA2R1 are associated with the development of the disease (45). Finally, we showed that the amount of mucin in the epithelium was decreased after methacholine challenge in the Pla2r1−/− mice, consistent with increased mucin release that contributes to AHR (38).
Our study is limited by the inability to determine cell-specific effects to further understand the function of epithelial Pla2r1 relative to leukocyte Pla2r1 on allergic sensitization, airway inflammation, airway edema, and AHR. The effects on allergic sensitization also need further exploration in models of allergic sensitization that have greater dependence on innate cells such as macrophages. The PLA2R1 receptor is a paralog of the mannose receptor, and has carbohydrate recognition domains that could be important in the recognition of components of common antigens, such as house dust mites. Effects on airway remodeling should also be explored in future model systems, as the expression of this receptor on myofibroblasts is important in the remodeling of the myocardium after an ischemic event (46), and we found that the peribronchial area is altered in Pla2r1−/− mice.
We conclude that PLA2R1 is overexpressed in the airway epithelium in asthma, and likely serves in the airways to reduce the effects of sPLA2s. In a murine model of asthma, the absence of the receptor led to a predominance of effects that increased cellular inflammation, peribronchial remodeling, and allergic sensitization. Furthermore, the absence of the receptor had pronounced effects on AHR. These results suggest that PLA2R1 plays an important role in the regulation of airway inflammation and airway edema relevant to asthma pathogenesis.
Footnotes
This work was supported by National Institutes of Health grants R01HL089215 (T.S.H.) and R37HL036235 (M.H.G.).
Author Contributions: A.K., G.L., W.R.H., M.H.G., and T.S.H. participated in conception and design of the research; J.D.N., H.L.O., Y.L., W.A.A., C.W.F., J.G.B., G.S.N., A.K., S.M.S., and T.S.H. performed the experiments; J.D.N. and T.S.H. analyzed data; J.D.N., H.L.O., W.A.A., C.W.F., A.K., W.R.H., M.H.G., and T.S.H. interpreted the results of the experiments; J.D.N., H.L.O., W.A.A., C.W.F., W.R.H., M.H.G., and T.S.H. edited and revised the manuscript; all authors approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0150OC on July 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hallstrand TS, Moody MW, Aitken ML, Henderson WR., Jr Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;116:586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, Godard P, Chanez P. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20:1370–1377. doi: 10.1183/09031936.02.00029202. [DOI] [PubMed] [Google Scholar]
- 3.Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am J Respir Crit Care Med. 2006;173:718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson WR, Jr, Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, Jones F, Eriksen P, Tien YT, Jonas M, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 6.Balboa MA, Shirai Y, Gaietta G, Ellisman MH, Balsinde J, Dennis EA. Localization of group V phospholipase A2 in caveolin-enriched granules in activated P388D1 macrophage–like cells. J Biol Chem. 2003;278:48059–48065. doi: 10.1074/jbc.M305904200. [DOI] [PubMed] [Google Scholar]
- 7.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2-α. J Biol Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 8.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 10.Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007;176:1072–1078. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallstrand TS, Lai Y, Ni Z, Oslund RC, Henderson WR, Jr, Gelb MH, Wenzel SE. Relationship between levels of secreted phospholipase A₂ groups IIA and X in the airways and asthma severity. Clin Exp Allergy. 2011;41:801–810. doi: 10.1111/j.1365-2222.2010.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallstrand TS, Lai Y, Altemeier WA, Appel CL, Johnson B, Frevert CW, Hudkins KL, Bollinger JG, Woodruff PG, Hyde DM, et al. Regulation and function of epithelial secreted phospholipase A2 group X in asthma. Am J Respir Crit Care Med. 2013;188:42–50. doi: 10.1164/rccm.201301-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson WR, Jr, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, et al. Importance of group X–secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson WR, Jr, Oslund RC, Bollinger JG, Ye X, Tien YT, Xue J, Gelb MH. Blockade of human group X secreted phospholipase A2 (GX-sPLA2)–induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective GX-sPLA2 inhibitor. J Biol Chem. 2011;286:28049–28055. doi: 10.1074/jbc.M111.235812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson WR, Jr, Ye X, Lai Y, Ni Z, Bollinger JG, Tien YT, Chi EY, Gelb MH. Key role of group V secreted phospholipase A2 in Th2 cytokine and dendritic cell–driven airway hyperresponsiveness and remodeling. PLoS One. 2013;8:e56172. doi: 10.1371/journal.pone.0056172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannattasio G, Fujioka D, Xing W, Katz HR, Boyce JA, Balestrieri B. Group V secretory phospholipase A2 reveals its role in house dust mite–induced allergic pulmonary inflammation by regulation of dendritic cell function. J Immunol. 2010;185:4430–4438. doi: 10.4049/jimmunol.1001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohta S, Imamura M, Xing W, Boyce JA, Balestrieri B. Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation. J Immunol. 2013;190:5927–5938. doi: 10.4049/jimmunol.1203202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese C, Triggiani M, Marone G, Mazzarella G. Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma. Allergy. 2000;55:27–30. doi: 10.1034/j.1398-9995.2000.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 21.Murakami M, Taketomi Y, Miki Y, Sato H, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A enzymes: the 3rd edition. Biochimie. 2014;107:105–113. doi: 10.1016/j.biochi.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Rouault M, Le Calvez C, Boilard E, Surrel F, Singer A, Ghomashchi F, Bezzine S, Scarzello S, Bollinger J, Gelb MH, et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 2007;46:1647–1662. doi: 10.1021/bi062119b. [DOI] [PubMed] [Google Scholar]
- 23.Zelensky AN, Gready JE. The C-type lectin–like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 24.Ancian P, Lambeau G, Mattéi MG, Lazdunski M. The human 180-kDa receptor for secretory phospholipases A2: molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem. 1995;270:8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 25.Fonteh AN, Atsumi G, LaPorte T, Chilton FH. Secretory phospholipase A2 receptor-mediated activation of cytosolic phospholipase A2 in murine bone marrow–derived mast cells. J Immunol. 2000;165:2773–2782. doi: 10.4049/jimmunol.165.5.2773. [DOI] [PubMed] [Google Scholar]
- 26.Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, Gelb MH, Lambeau G, Marone G, Triggiani M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–474. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 27.Mandal AK, Zhang Z, Chou JY, Mukherjee AB. Pancreatic phospholipase A2 via its receptor regulates expression of key enzymes of phospholipid and sphingolipid metabolism. FASEB J. 2001;15:1834–1836. doi: 10.1096/fj.00-0831fje. [DOI] [PubMed] [Google Scholar]
- 28.Hanasaki K, Arita H. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 2002;68-69:71–82. doi: 10.1016/s0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 29.Yokota Y, Notoya M, Higashino K, Ishimoto Y, Nakano K, Arita H, Hanasaki K. Clearance of group X secretory phospholipase A2 via mouse phospholipase A2 receptor. FEBS Lett. 2001;509:250–254. doi: 10.1016/s0014-5793(01)03173-8. [DOI] [PubMed] [Google Scholar]
- 30.Zvaritch E, Lambeau G, Lazdunski M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J Biol Chem. 1996;271:250–257. doi: 10.1074/jbc.271.1.250. [DOI] [PubMed] [Google Scholar]
- 31.Tamaru S, Mishina H, Watanabe Y, Watanabe K, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata K, et al. Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J Immunol. 2013;191:1021–1028. doi: 10.4049/jimmunol.1300738. [DOI] [PubMed] [Google Scholar]
- 32.Kicic A, Hallstrand TS, Sutanto EN, Stevens PT, Kobor MS, Taplin C, Paré PD, Beyer RP, Stick SM, Knight DA. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am J Respir Crit Care Med. 2010;181:889–898. doi: 10.1164/rccm.200907-1071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62:693–697. doi: 10.1369/0022155414545224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates JH, Wagers SS, Norton RJ, Rinaldi LM, Irvin CG. Exaggerated airway narrowing in mice treated with intratracheal cationic protein. J Appl Physiol (1985) 2006;100:500–506. doi: 10.1152/japplphysiol.01013.2005. [DOI] [PubMed] [Google Scholar]
- 36.McNamee EN, Wermers JD, Masterson JC, Collins CB, Lebsack MD, Fillon S, Robinson ZD, Grenawalt J, Lee JJ, Jedlicka P, et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm Bowel Dis. 2010;16:743–752. doi: 10.1002/ibd.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilic D, Bollinger JM, Gelb M, Mauro TM. sPLA2 and the epidermal barrier. Biochim Biophys Acta. 2014;1841:416–421. doi: 10.1016/j.bbalip.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas U, Podda M, Behne M, Gurrieri S, Alonso A, Fürstenberger G, Pfeilschifter J, Lambeau G, Gelb MH, Kaszkin M. Characterization and differentiation-dependent regulation of secreted phospholipases A in human keratinocytes and in healthy and psoriatic human skin. J Invest Dermatol. 2005;124:204–211. doi: 10.1111/j.0022-202X.2004.23513.x. [DOI] [PubMed] [Google Scholar]
- 41.Mulherkar R, Kirtane BM, Ramchandani A, Mansukhani NP, Kannan S, Naresh KN. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene. 2003;22:1936–1944. doi: 10.1038/sj.onc.1206229. [DOI] [PubMed] [Google Scholar]
- 42.Augert A, Vindrieux D, Girard CA, Le Calvé B, Gras B, Ferrand M, Bouchet BP, Puisieux A, de Launoit Y, Simonnet H, et al. PLA2R1 kills cancer cells by inducing mitochondrial stress. Free Radic Biol Med. 2013;65:969–977. doi: 10.1016/j.freeradbiomed.2013.08.177. [DOI] [PubMed] [Google Scholar]
- 43.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Southam DS, Ellis R, Wattie J, Inman MD. Components of airway hyperresponsiveness and their associations with inflammation and remodeling in mice. J Allergy Clin Immunol. 2007;119:848–854. doi: 10.1016/j.jaci.2006.12.623. [DOI] [PubMed] [Google Scholar]
- 45.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 46.Mishina H, Watanabe K, Tamaru S, Watanabe Y, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata K, et al. Lack of phospholipase A2 receptor increases susceptibility to cardiac rupture after myocardial infarction. Circ Res. 2014;114:493–504. doi: 10.1161/CIRCRESAHA.114.302319. [DOI] [PubMed] [Google Scholar]