Abstract
Patients with progressive sarcoidosis exhibit increased expression of programmed death-1 (PD-1) receptor on their CD4+ T cells. Up-regulation of this marker of T cell exhaustion is associated with a reduction in the proliferative response to T cell receptor (TCR) stimulation, a defect that is reversed by PD-1 pathway blockade. Genome-wide association studies and microarray analyses have correlated signaling downstream from the TCR with sarcoidosis disease severity, but the mechanism is not yet known. Reduced phosphatidylinositol 3-kinase (PI3K)/AKT expression inhibits proliferation by inhibiting cell cycle progression. To test the hypothesis that PD-1 expression attenuates TCR-dependent activation of PI3K/AKT activity in progressive systemic sarcoidosis, we analyzed PI3K/AKT/mechanistic target of rapamycin (mTOR) expression at baseline and after PD-1 pathway blockade in CD4+ T cells isolated from patients with sarcoidosis and healthy control subjects. We confirmed an increased percentage of PD-1+ CD4+ T cells and reduced proliferative capacity in patients with sarcoidosis compared with healthy control subjects (P < 0.001). There was a negative correlation with PD-1 expression and proliferative capacity (r = −0.70, P < 0.001). Expression of key mediators of cell cycle progression, including PI3K and AKT, were significantly decreased. Gene and protein expression levels reverted to healthy control levels after PD-1 pathway blockade. Reduction in sarcoidosis CD4+ T cell proliferative capacity is secondary to altered expression of key mediators of cell cycle progression, including the PI3K/AKT/mTOR pathway, via PD-1 up-regulation. This supports the concept that PD-1 up-regulation drives the immunologic deficits associated with sarcoidosis severity by inducing signaling aberrancies in key mediators of cell cycle progression.
Keywords: programmed death-1, sarcoidosis, proliferation, lymphocyte cell-specific protein-tyrosine kinase, phosphatidylinositol 3-kinase, AKT
Clinical Relevance
Sarcoidosis is a granulomatous disease characterized by spontaneous T helper cell type 1 cytokine production but reduced cytokine secretion and proliferative capacity succeeding T cell receptor stimulation among subjects experiencing disease progression. This is the first investigation to demonstrate the specific T cell receptor signaling mediators that play a critical role in the loss of sarcoidosis proliferative capacity through programmed death (PD)-1 intervention. Specifically, we demonstrate that PD-1 strategically targets a key tyrosine kinase, lymphocyte cell-specific protein-tyrosine kinase, and effectively prevents signaling through the phosphatidylinositol 3-kinase/AKT/mechanistic target of rapamycin pathway, a crucial pathway for cell cycle progression and IL-2 transcription (cytokine that promotes lymphocyte proliferation), thereby affecting sarcoidosis T cell proliferation. More importantly, this work is the first to reveal normalization of these molecular mediators of proliferation after PD-1 pathway blockade in patients with sarcoidosis.
Sarcoidosis is a granulomatous disease characterized by spontaneous secretion of Th1 cytokines, but reduced cytokine expression and proliferative capacity after T cell receptor (TCR) stimulation among subjects experiencing disease progression (1, 2).
TCR activation is an organized and intricate process that integrates both extracellular and intracellular signals. Stimulation of the TCR/CD3 complex together with costimulatory molecules, such as CD28, promotes T cell activation and cytokine secretion, and directly regulates cell cycle progression (3–5). CD28 has been shown to mediate cell cycle progression via activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B, also known as AKT, signaling pathway (6). Activation of the TCR and engagement of CD28 by its ligand results in the association of CD28 with the Src Homology 2 (SH2) domain of PI3K, located in its p85 subunit (7).
Programmed death (PD)-1 signaling inhibits AKT activation by impeding CD28-mediated phosphorylation of PI3K (8). Furthermore, PD-1 engagement has also been shown to suppress CD4+ T cell proliferation by repressing TCR-induced activation of the PI3K/AKT pathway and by suppressing S-phase kinase-associated protein 2 (Skp2) transcription (9). The importance of lymphocyte cell-specific protein-tyrosine kinase (LCK) activity for initiation of TCR signaling has been established by several investigations (10–12). After TCR engagement, the sarcoma kinase, p56LCK (LCK), is responsible for phosphorylating sequences of the immunoreceptor tyrosine-based activation motif in the TCR–CD3 complex (11). Mice with an LCK deficiency in systemic T lymphocytes have been reported to exhibit diminished proliferative capacity (13).
We report for the first time that PD-1 is a key regulator of the deficient TCR signaling and reduced proliferative capacity linked to sarcoidosis severity. Analysis of sarcoidosis CD4+ T cells with impaired proliferative capacity reveals PD-1 up-regulation with reduced PI3K/AKT/mechanistic target of rapamycin (mTOR) expression. This expression defect was completely eliminated with PD-1 pathway blockade, and was accompanied by return to normal proliferative capacity.
Materials and Methods
Study Population
Patients with sarcoidosis were diagnosed at Vanderbilt University Medical Center (Nashville, TN) according the American Thoracic Society/World Association for Sarcoidosis and Other Granulomatous Disorders (WASOG) clinical, histological, and radiographic diagnostics. All subjects provided written informed consent approved by the Vanderbilt Institutional Review Board. Study participants were categorized solely based upon CD4+ T cell proliferative capacities of 50% or greater (“normal”; ranges analogous to healthy control subjects) and less than 50% (“impaired”). This cut off was based upon prior publications demonstrating proliferative capacities of less than 50% with sarcoidosis disease severity (1, 2). Participant demographics are outlined in Table 1. Table 2 summarizes demographics for the subjects with sarcoidosis characterized by their proliferation phenotype.
Table 1.
Sarcoidosis and Healthy Control Demographic Information
| Characteristics | Patients with Sarcoidosis | Healthy Control Subjects |
|---|---|---|
| n | 38 | 26 |
| Sex (female/male), n | 22/16 | 14/12 |
| Age, median (min, max), yr | 47 (20, 67) | 35 (22, 67) |
| Race, n | 19 W/19 AA | 19 W/6 AA/1 AI |
Definition of abbreviations: AA, African American; AI, American Indian; W, white.
Table 2.
Sarcoidosis Normal versus Impaired Proliferation Demographic Information
| Characteristics | Normal | Impaired |
|---|---|---|
| n | 17 | 21 |
| Sex (female/male), n | 12/5 | 10/11 |
| Age, median (min, max), yr | 50 (24, 65) | 46 (20, 67) |
| Race, n | 9 W/8 AA | 10 W/11 AA |
Definition of abbreviations: AA, African American; W, white.
Cell Isolation
Peripheral blood was processed as previously described (14, 15). CD4+ T cells were isolated from peripheral blood mononuclear cells using Dynabeads CD4+ isolation kit (Invitrogen, Grand Island, NY) according to the manufacturer’s protocol.
Cell Cycle Progression Analysis
Actively cycling CD4+ T cells from healthy control subjects and patients with sarcoidosis were determined by in vitro labeling with bromodeoxyuridine (BrdU). After fixation, cells were stained with conjugated antibodies against BrdU and counterstained with 7-AAD, followed by flow cytometric analysis with BD Pharmingen BrdU Flow Kits (BD Biosciences, San Jose, CA), according to the manufacturer’s instructions.
Flow Cytometry
Antibodies specific for CD3, CD4, CD45RO, cytokine–cytokine receptor (CCR) 7, CD25, CD127, CCR4, CCR6, CXCR3, forkhead box P3, and PD-1 (BD Biosciences, San Jose, CA) were used for surface staining of cells as previously described (1). All experiments were carried out with an LSR-II flow cytometer (BD Biosciences), with a minimum of 100,000 events per sample. Calibrator beads were used to calibrate the FACS machine before each run. Cells were gated on live cells based on forward- and side-scatter properties. Cells were gated on singlets, CD3+, and CD4+ populations, and then analyzed using FlowJo X software (Tree Star, Ashland, OR).
Proliferation Assay and In Vitro Blockade of PD-1 Pathway
For the blockade experiment, peripheral blood mononuclear cells were labeled with carboxyfluorescein succinimidyl ester as previously described (1), then incubated overnight with or without the combination of anti–PD-1 (5 μg/ml), anti–PD-ligand 1 (2 μg/ml), and anti–PD-ligand 2 (2 μg/ml) in RPMI 1640–supplemented medium before stimulation with anti-CD3 (OKT-3) and anti-CD28 (1 μg/ml; BD Biosciences) antibodies at a final concentration of 2 × 106/ml for 5 days, 5% CO2 atmosphere.
RNA Isolation and Quantitative RT-PCR
Total cellular RNA was extracted from purified, resting CD4+ T cells or after 5-day TCR stimulation, then cDNA was generated as previously described (2). Quantitative RT-PCR amplification was performed in triplicate using 2× TaqMan Universal PCR Mastermix (Applied Biosystems/Life Technologies, Foster City, CA) and TaqMan gene expression assays targeting programmed cell death 1 (PDCD1) PDCD1, LCK, PIK3CD, AKT, and MTOR (TaqMan gene expression assays; Applied Biosystems/Life Technologies). Gene expression levels were normalized to β-actin and glyceraldehyde phosphate dehydrogenase. All reactions were performed in a StepOnePlus Real Time PCR System (Applied Biosystems).
Lysates, SDS-PAGE, and Western Blotting
CD4+ T cells were TCR stimulated and lysed as described previously (9). Cell lysates were resolved by SDS-PAGE and then analyzed by Western blotting. Band visualization and densitometry was completed using a Li-COR Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) and studio software. For more detailed information, see the supplemental Materials and Methods section.
Statistical Analysis
Comparisons between cohorts were performed using an unpaired, two-tailed Student’s t test. Multiple group comparisons were performed using a one-way ANOVA. Proliferation data were analyzed using the Mann–Whitney U test. Pearson’s correlation was used to determine relationships. Statistical analysis for all figures was performed using Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA). A P value <0.05 was considered statistically significant.
Results
PD-1 Up-Regulation on Sarcoidosis CD4+ T Cells Strongly Correlates with Loss of Proliferative Capacity
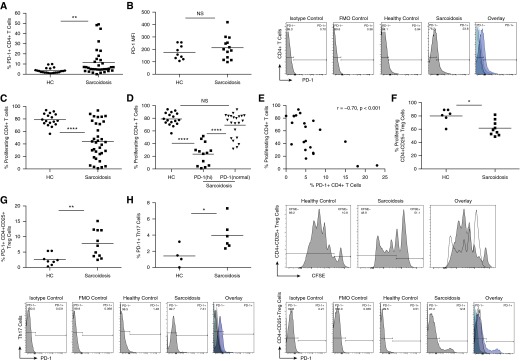
Sarcoidosis CD4+ T cells exhibit reduced proliferative capacity upon TCR stimulation, compared with healthy controls (1, 2). It was also noted that blockade of the PD-1 pathway restored proliferative capacity in sarcoidosis CD4+ T cells (1). Prior reports have demonstrated that the degree of PD-1 up-regulation on T cells is a contributor to the manifestation of immune dysfunction (16). We began by examining PD-1 expression on healthy control and sarcoidosis CD4+ T cells. A significantly greater percentage of sarcoidosis CD4+ T cells expressed PD-1 than did healthy controls (P = 0.0063, two-tailed t test; Figure 1A). We also assessed for median fluorescent intensity on CD4+ T cells from both cohorts. The PD-1 median fluorescent intensity was not significantly higher on sarcoidosis CD4+ T cells than on healthy controls (P = 0.30; Figure 1B). Comparing proliferation potential between subjects with sarcoidosis and healthy control subjects, we observed significantly lower proliferative capacity among subjects with sarcoidosis (P < 0.0001; Figure 1C). In addition, percent proliferation was compared among patients with sarcoidosis expressing high and normal levels of PD-1 (within healthy control range; Figure 1D). We investigated the correlation of PD-1 expression on sarcoidosis CD4+ T cells with proliferation and noted that the percentage of cells expressing PD-1 correlated significantly with loss of proliferative capacity (Figure 1E; r = −0.70; P < 0.001). CD4+ T cell subsets were analyzed for PD-1 receptor expression as well as proliferation. Decreased proliferation was noted on sarcoidosis CD4+ CD25+ T regulatory cells compared with that on healthy controls (sarcoidosis, n = 8; healthy control, n = 6; P = 0.0196). Both sarcoidosis CD4+ CD25+ T regulatory cells and Th17 cells exhibited an up-regulation in PD-1 receptor expression compared with the controls (P = 0.008, P = 0.0.0448, respectively). A correlation analysis between PD-1 receptor expression and proliferation of sarcoidosis CD4+ CD25+ T regulatory cells revealed a significance of 0.0283 (r = −0.6865; data not shown).
Figure 1.
Strong negative correlation between programmed death (PD)-1 plus CD4+ T cells and proliferation in patients with sarcoidosis. (A) Percent total peripheral CD4+ T cells expressing PD-1 from healthy control subjects (HCs) (n = 22) and patients with sarcoidosis (n = 34). (B) Median fluorescent intensity (MFI) of systemic CD4+ T cells expressing PD-1 in HC (n = 9) and subjects with sarcoidosis (n = 13) (P > 0.05). Representative histograms depicting PD-1 levels for control subjects and a sarcoidosis patient. (C) Percent total peripheral CD4+ T cells proliferating from HCs (n = 17) and patients with sarcoidosis (n = 34) after in vitro T cell receptor (TCR) stimulation. (D) Proliferation percentages categorized according to high PD-1 or normal PD-1 expression levels. (E) Linear correlation between percent PD-1 expression and proliferating CD4+ T cells from patients with sarcoidosis. (F) Percent of proliferating systemic CD4+ CD25+ T regulatory cells from HCs (n = 6) and subjects with sarcoidosis (n = 8) after TCR stimulation with plate-bound anti-CD3 and anti-CD28. (G) Baseline PD-1 expression in peripheral CD4+ CD25+ T regulatory cells and (H) systemic T helper type 17 (Th17) cells (HC; n = 4; sarcoidosis, n = 6). *P < 0.05, **P < 0.01, ****P < 0.0001; CFSE, carboxyfluorescein succinimidyl ester; FMO, fluorescence minus one; NS, not significant (P > 0.05).
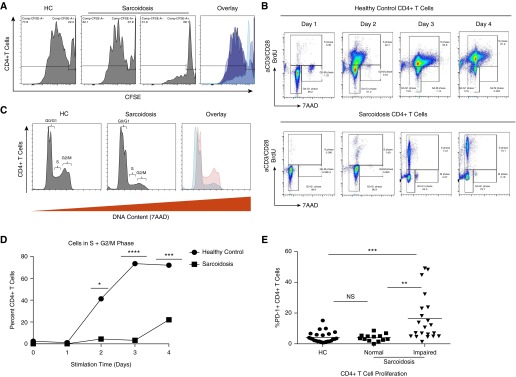
Cellular proliferation reflects an increase in cell numbers due to cell growth and division. Investigation of cellular proliferation revealed patients with reduced numbers of proliferating CD4+ T cells, in addition to subjects demonstrating normal (levels comparable to healthy control subjects) proliferation (Figure 2A). To confirm that the observed difference in proliferation was due to decreased rate of cellular division and not increased cell death, we performed a cell cycle assay. Flow cytometric analysis of BrdU incorporation and DNA content revealed that sarcoidosis CD4+ T cells did not progress from the G0/G1 phase to the S phase, and even less to the G2/M phase (Figure 2B). Overall, significantly fewer sarcoidosis CD4+ T cells demonstrated progression through the terminal phase of the cell cycle compared with healthy control CD4+ T cells (Figures 2C and 2D). Closer inspection of these cohorts revealed PD-1 expression to be significantly elevated in sarcoidosis CD4+ T cells with impaired proliferation and cell cycle progression when compared with healthy controls (P < 0.001; Figure 2E); this distinction in PD-1 expression was not apparent in subjects with sarcoidosis with normal proliferative capacity when compared with healthy control subjects (P = 0.8531; Figure 2E).
Figure 2.
Sarcoidosis CD4+ T cells do not exhibit normal progression into the S phase of the cell cycle. Peripheral CD4+ T cells were TCR stimulated by cross-linking with plate-bound anti-CD3 antibody (Ab) and soluble anti-CD28 Ab. At Day 5 postactivation, cells were fixed and analyzed for CFSE expression by flow cytometry. (A) Representative histograms illustrating proliferation levels after 5-day in vitro stimulation for an HC, as well as a subject with sarcoidosis with normal and one with impaired proliferation. (B–D) CD4+ T cells were cultured with bromodeoxyuridine (BrdU) preceding TCR stimulation for the indicated time points, after which cell cycle progression of five HCs and seven patients with sarcoidosis was analyzed using flow cytometry. Percentages of cells in G0/G1, S and G2/M phase. (E) Percent PD-1 expression in peripheral CD4+ T cells from HCs (n = 25) and patients with sarcoidosis categorized according to their proliferative phenotype (normal [>50%], n = 13; impaired [<50%], n = 21). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; NS, not significant (P > 0.05). 7AAD, 7-aminoactinomycin D.
Reduced Expression of the PI3K/AKT Pathway Is Present within Sarcoidosis CD4+ T Cells with Impaired Proliferative Capacity
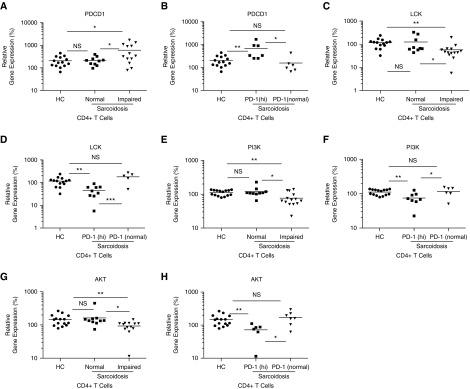
After TCR stimulation, the tyrosine kinase, LCK, is immediately recruited to the immunological synapse (17). After its activation, key downstream signaling events involving protein kinases, such as PI3K and its target AKT, are critical for the initiation of cellular proliferation (18). We hypothesized that disruption of these signaling pathways would account for the proliferative defect of sarcoidosis T cells. We therefore assessed the expression of PDCD1, LCK, PI3K, and AKT in CD4+ T cells from healthy control subjects, patients with sarcoidosis with impaired CD4+ T proliferative capacity, and patients with sarcoidosis with normal T cell proliferation. There were increased PDCD1 expression levels in sarcoidosis CD4+ T cells with reduced proliferation compared with both healthy subjects (P = 0.01) and patients with sarcoidosis with normal proliferation (P = 0.03) (Figure 3A). Sarcoidosis CD4+ T cells from subjects with normal proliferation did not yield statistically significant differences from those from healthy control subjects (P = 0.87; Figure 3A). We observed significantly reduced LCK, PI3K, and AKT expression in the sarcoidosis CD4+ T cells with impaired proliferation compared with those of both healthy controls (P < 0.01) and from subjects with sarcoidosis with normal proliferation (P = 0.04, P = 0.01, P = 0.03, respectively; Figures 3C, 3E, and 3G). Importantly, there were no detectable differences between the healthy cohorts and normally proliferating sarcoidosis CD4+ T cells for any of the genes (P = 0.80, P = 0.50, P = 0.59, respectively; Figures 3C, 3E, and 3G). Furthermore, a comparison between the impaired sarcoidosis CD4+ T cells expressing high PD-1 and normal PD-1 levels was made (Figures 3B, 3D, 3F, and 3H).
Figure 3.
Reduced lymphocyte cell-specific protein tyrosine kinase (LCK), phosphatidylinositol 3-kinase (PI3K), and AKT expression in sarcoidosis CD4+ T cells with impaired proliferation. (A–H) RNA was isolated from unstimulated sorted peripheral CD4+ T cells, cDNA was synthesized, and gene expression analysis conducted using quantitative RT-PCR (qRT-PCR). PDCD1, LCK, PI3K, and AKT were normalized to an HC and glyceraldehyde phosphate dehydrogenase. Data represent 15 HCs and 13 patients with sarcoidosis with normal proliferative capacity, as well as 15 with abnormal proliferation. Subjects with impaired proliferative capacity were further subcategorized into CD4+ T cells expressing high PD-1 (“PD-1[hi]”) and normal PD-1 (“PD-1[normal]”) levels. *P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant; PDCD1, programmed cell death 1 gene (P > 0.05).
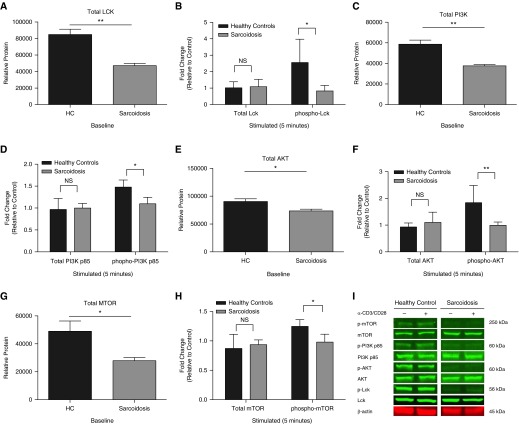
To examine the functional activity of LCK, PI3K, AKT, and mTOR kinases in sarcoidosis CD4+ T cells with reduced proliferation, we quantified phosphorylation by Western blotting using anti-phosphotyrosine antibodies. Compared with healthy control subjects (n = 5), subjects with sarcoidosis with impaired proliferation (n = 5) demonstrated a significant reduction of phosphorylation of LCK on tyrosine 394 (Y394), which marks the activated form of the kinase (P = 0.029; Figure 4B). Significant reductions in PI3K p85 (Y458/Y199), as well as AKT threonine 308 (T308) and mTOR serine 2,448 (S2448) were also detected (Figures 4D, 4F, and 4H; P = 0.022, P < 0.01, P = 0.0240, respectively). A significant decrease in total protein levels at baseline (no stimulation) in sarcoidosis CD4+ T cells was revealed compared with controls: LCK (P < 0.01), PI3K (P < 0.01), AKT (P = 0.0253), and mTOR (P = 0.0340) (Figures 4A, 4C, 4E, and 4G).
Figure 4.
Sarcoidosis CD4+ T cells with impaired proliferation demonstrate reduced LCK, PI3K, AKT, and mechanistic target of rapamycin (mTOR) protein phosphorylation. Purified systemic CD4+ T cells from HCs and patients with sarcoidosis were anti-CD3/CD28 stimulated for 5 minutes. LCK, PI3K, AKT, and mTOR protein activation was assessed in HCs (n = 5) and patients with sarcoidosis (n = 5) with impaired proliferation after TCR stimulation. (A–H) Using specific antibodies, total and phosphorylated LCK (tyrosine 394 [Y394]), PI3K p85 (tyrosine 467/199 [Y467/199]), AKT (threonine 308 [T308]), and mTOR (serine 2,448 [S2448]) at their activation sites were detected after stimulation using Western blot analysis. Error bars show SEM. *P < 0.05, **P < 0.01; NS, not significant (P > 0.05). (I) Representative blots for an HC and patient with sarcoidosis.
Significant Correlations between PD-1 Receptor Expression and the PI3K/AKT Pathway Gene Expression
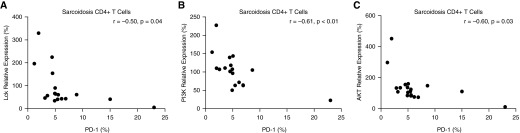
After the identification of reduced expression of key mediators of cellular proliferation in the PI3K/AKT pathway, we then compared gene expression data to PD-1 receptor levels within the same subjects with sarcoidosis using Pearson’s correlation coefficient. The findings suggest a significant negative correlation between the LCK gene and PD-1 receptor expression (r = −0.50, P = 0.04; Figure 5A). A negative correlation was also apparent for both PI3K and AKT gene expression and the PD-1 receptor (r = −0.61, P < 0.01; r = −0.60, P = 0.03, respectively; Figures 5B and 5C).
Figure 5.
Strong negative correlations between PD-1 receptor expression and gene relative expression in sarcoidosis CD4+ T cells. Percent relative gene expression for LCK, PI3K, and AKT in sarcoidosis CD4+ T cells in relation to PD-1 (n = 17). CD4+ T cells were purified from peripheral blood mononuclear cells from patients with sarcoidosis and gene expression analysis performed using qRT-PCR, as well as PD-1 levels determined using flow cytometry. Percent PD-1 expressed on total sarcoidosis CD4+ T cells in relation to (A) LCK, (B) PI3K, and (C) AKT was analyzed using Pearson’s correlation.
Blockade of PD-1 Pathway Restores PI3K/AKT Signaling in Sarcoidosis CD4+ T Cells with Reduced Proliferative Capacity
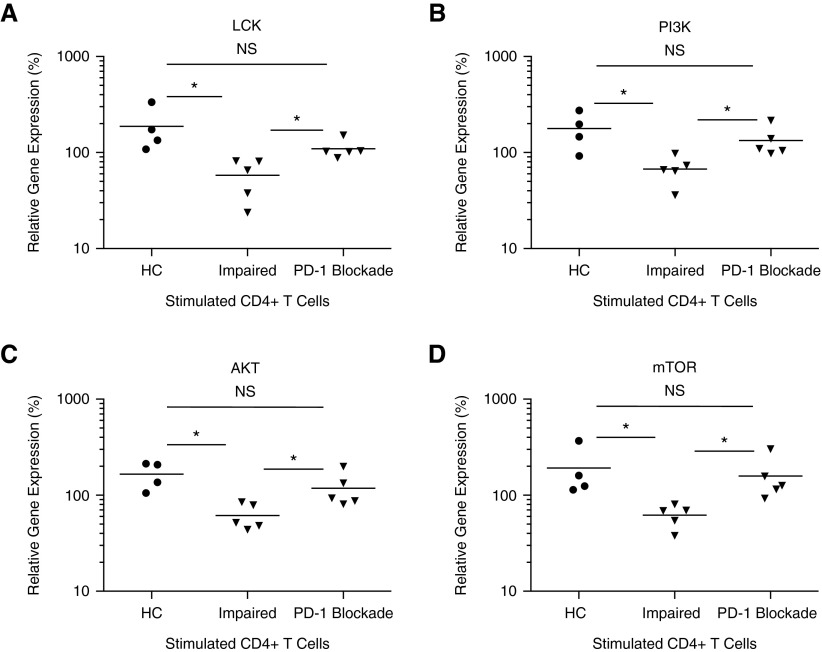
To assess if PD-1 up-regulation mediated the reductions in PI3K/AKT signaling that we detected, we examined their gene levels in sarcoidosis CD4+ T cells after PD-1 pathway blockade. As previously observed, reduced LCK expression was noted between healthy controls and sarcoidosis T cells with impaired proliferation (P = 0.02; Figure 6A). Remarkably, PD-1 pathway blockade completely eliminated the difference between healthy and sarcoidosis CD4+ T cells (P = 0.13; Figure 6A). Diminished PI3K and AKT expression was also observed in sarcoidosis CD4+ T cells with abnormal proliferation before PD-1 pathway blockade (P = 0.01 and P < 0.01; Figures 6B and 6C). Expression levels were restored to healthy levels after blockade (P = 0.33 and P = 0.21, respectively; Figures 6B and 6C). MTOR gene expression was also reduced in sarcoidosis CD4+ T cells with reduced proliferation, but was restored to levels akin to CD4+ T cells of healthy controls after PD-1 blockade (P = 0.04, preblockade; P = 0.64, postblockade; Figure 6D).
Figure 6.
Blockade of PD-1 pathway restores gene expression. (A–D) Purified peripheral CD4+ T cells from HCs (n = 4) and patients with sarcoidosis (n = 5) were stimulated by cross-linking with plate-bound anti-CD3 and anti-CD28 Ab and cultured for 5 days in RPMI 1640–supplemented medium succeeding overnight incubation with or without PD-1–blocking Ab. Cells were then collected and gene expression analysis for LCK, PI3K, AKT, and MTOR was performed using qRT-PCR. *P < 0.05; NS, not significant (P > 0.05).
Discussion
Prior genome-wide association studies (GWASs) and microarray analyses demonstrate that the PI3K/AKT signaling pathway contributes to sarcoidosis severity, yet the mechanism(s) by which this occurs are not known. Independent GWAS analyses implicate aberrancies in TCR signaling, janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling, and CCR signaling in sarcoidosis severity. GWAS revealed a significant association of single-nucleotide polymorphisms in signature genes with sarcoidosis susceptibility and severity (unbiased signature genes: CX3CR1, FKBP1A, NOG, RBM12B, SENS3, TSHZ2; T cell/JAK/STAT pathway genes, such as: AKT3, CBLB, DLG1, IFNG, IL2RA, IL7R, ITK, JUN, MALT1, NFATC2, PLCG1, SPRED1, as well as those associated with IL23/Th17 signaling) (19, 20). Ingenuity pathway analysis to a cross-sectional derivation microarray dataset identified up-regulation of genes related to IFN signaling and down-regulation of TCR signaling pathways in severe sarcoidosis. CXCL9 and TCR factors discriminated between chronic versus nonprogressive disease, and CXCL9 predicted disease outcomes longitudinally. Factors associated with lung function decline included decreased TCR factor and increased CXCL9. Down-regulation of the TCR signaling pathway was associated with severity, as manifested by lung function decline (21).
This investigation demonstrates that sarcoidosis CD4+ T cells do not have normal cell cycle progression, but rather demonstrate blocked progression through the G1 phase, resulting in reduced proliferation (Figure 2). Although there are numerous mechanisms by which PD-1 affects T cell proliferation, prior studies have demonstrated that PD-1 impedes cell cycle progression through the G1 phase by suppressing transcription of the ubiquitin ligase, Skp1/Cul1/Skp2 (SCFSkp2), which encodes a component of this ubiquitin ligase. PD-1 suppresses SKP2 transcription by inhibiting PI3K/AKT signaling (9). PD-1 up-regulation also strategically inhibits induction of a key tyrosine kinase, p56LCK, which effectively decreases signaling through the PI3K/AKT pathway. Quantitative RT-PCR analysis for the relative expression of this pathway confirmed reduced expression of PI3K/AKT effectors in sarcoidosis CD4+ T cells with impaired proliferative capacity and high PD-1 expression, whereas expression was normal in healthy control subjects and in subjects with sarcoidosis with normal proliferative capacity (Figure 3). Equally encouraging, the PI3K/AKT transcription defect was rescued by PD-1 pathway blockade (Figure 6), with resultant increases in CD4+ T cell proliferative capacity. This finding has potential for therapeutic translation.
A clear understanding of how enhanced immune function, specifically proliferative capacity, contributes to improvement in sarcoidosis clinical outcome is not intuitive. Numerous elegant studies demonstrate that sarcoidosis T cells have increased cytokine production, as well as evidence of TCR stimulation (22, 23). These studies have been complemented by molecular analyses demonstrating that microbial antigens are present within sarcoidosis granulomas (24–28) and that these same antigens are targets of the sarcoidosis adaptive immune response (14, 26, 29–31). The expression of Th1 cytokines is the healthy adaptive immune response attempt to signal immune mediators that can clear foreign antigen, leading to its clearance. Healthy T cells also undergo a rapid, vast differentiation and expansion program in response to foreign antigens to facilitate their clearance. An important immune evasion mediated by foreign antigen is PD-1 up-regulation, and the resultant effects of reduced cytokine expression and proliferative capacity after TCR stimulation. This loss of proliferative capacity leads to impaired clearance of microbial antigens; this antigenic persistence leads to disease progression in some subjects with sarcoidosis (1, 2). Blockade of the PD-1 pathway not only resulted in immediate restoration of T cell proliferation, but also immediate induction of normal expression of the key mediators of cell cycle progression, including PI3K/AKT/mTOR (Figure 6).
Limitations of this analysis include that these results do not exclude the role of other negative regulatory molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), in loss of proliferative capacity. Although it does demonstrate one of the signaling mechanisms by which the PD-1 pathway mediates loss of cell cycle progression (i.e., proliferative capacity), it is not an exhaustive analysis. It is quite possible that other contributors to the loss of immune function are present. Previous reports demonstrate that the degree of immunosuppression did not play a role in the loss of sarcoidosis proliferative capacity, nor its restoration after PD-1 pathway blockade (1, 2).
In conclusion, these studies provide mechanistic insight into the relevant signaling mechanisms by which PD-1 ligation alters sarcoidosis CD4+ T cell proliferative capacity. The significant role that the PI3K/AKT pathway plays in oncogenic transformation has been well documented, and its involvement in cell cycle progression and cellular proliferation has been extensively investigated (32–35). PI3K inhibition has been shown to impede the growth of various cancers by attenuating cell proliferation and inducing G1 cell cycle arrest (36, 37). The inhibition of this pathway is an important strategy for reduced proliferation of malignant cells; it is therefore suboptimal for a healthy immune response against foreign antigen.
Footnotes
This work was supported by National Institutes of Health grants T32HL069765 (L.J.C.) and T32HL094296 (C.H.), and Pierce Foundation grants U01 HL112694-01, R01 HL117074, and UL1 RR-024975 (W.P.D.). The Vanderbilt University Medical Center Flow Cytometry Shared Resource is supported by Vanderbilt Ingram Cancer Center grant P30 CA68485 and Vanderbilt Digestive Disease Research Center grant DK058404.
Author Contributions: Conception and design—L.J.C. and W.P.D.; performing experiments—L.J.C., J.E.R., A.Y., G.S., D.S., and C.H.; analysis and interpretation—L.J.C., C.H., and W.P.D.; drafting the manuscript for important intellectual content—L.J.C. and W.P.D.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0037OC on August 26, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM, Grutters J, Culver DA, Dworski R, Sheller J, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4+ T-cell proliferative capacity. Am J Respir Crit Care Med. 2014;190:560–571. doi: 10.1164/rccm.201401-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oswald-Richter KA, Richmond BW, Braun NA, Isom J, Abraham S, Taylor TR, Drake JM, Culver DA, Wilkes DS, Drake WP. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J Immunol. 2013;190:5446–5453. doi: 10.4049/jimmunol.1202891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2–independent and IL-2–dependent regulation of cell cycle progression. J Immunol. 2000;164:144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 6.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 7.Pagès F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 8.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu K, Littman DR. Requirement for kinase activity of CD4-associated p56lck in antibody-triggered T cell signal transduction. J Biol Chem. 1994;269:24095–24101. [PubMed] [Google Scholar]
- 11.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 12.Rossy J, Owen DM, Williamson DJ, Yang Z, Gaus K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat Immunol. 2013;14:82–89. doi: 10.1038/ni.2488. [DOI] [PubMed] [Google Scholar]
- 13.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naïve T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 14.Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, Jenkins CA, Judson MA, Drake WP. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77:3740–3748. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oswald-Richter K, Sato H, Hajizadeh R, Shepherd BE, Sidney J, Sette A, Newman LS, Drake WP. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J Clin Immunol. 2010;30:157–166. doi: 10.1007/s10875-009-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 18.Xue L, Chiang L, Kang C, Winoto A. The role of the PI3K-AKT kinase pathway in T-cell development beyond the β checkpoint. Eur J Immunol. 2008;38:3200–3207. doi: 10.1002/eji.200838614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS, Ma SF, Wade MS, Noth I, Machado RF, et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS One. 2012;7:e44818. doi: 10.1371/journal.pone.0044818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer A, Ellinghaus D, Nutsua M, Hofmann S, Montgomery CG, Iannuzzi MC, Rybicki BA, Petrek M, Mrazek F, Pabst S, et al. GenPhenReSa Consortium. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am J Respir Crit Care Med. 2015;192:727–736. doi: 10.1164/rccm.201503-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J, Garudadri S, Ho M, Woodruff PG, Koth LL. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. Eur Respir J. 2014;44:985–993. doi: 10.1183/09031936.00039714. [DOI] [PubMed] [Google Scholar]
- 22.Saltini C, Spurzem JR, Lee JJ, Pinkston P, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest. 1986;77:1962–1970. doi: 10.1172/JCI112525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, Teirstein AS, Zhang Y, Cotter RJ, Moller DR. Mycobacterial catalase–peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oswald-Richter KA, Beachboard DC, Seeley EH, Abraham S, Shepherd BE, Jenkins CA, Culver DA, Caprioli RM, Drake WP. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol. 2012;32:1129–1140. doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen SS, Evans W, Carlisle J, Hajizadeh R, Nadaf M, Shepherd BE, Pride DT, Johnson JE, Drake WP. Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses. Respir Res. 2008;9:36. doi: 10.1186/1465-9921-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake WP, Pei Z, Pride DT, Collins RD, Cover TL, Blaser MJ. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis. 2002;8:1334–1341. doi: 10.3201/eid0811.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotsinger JE, Celada LJ, Polosukhin VV, Atkinson JB, Drake WP. Molecular analysis of sarcoidosis granulomas reveals antimicrobial targets. Am J Respir Cell Mol Biol. 2016;55:128–134. doi: 10.1165/rcmb.2015-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richmond BW, Ploetze K, Isom J, Chambers-Harris I, Braun NA, Taylor T, Abraham S, Mageto Y, Culver DA, Oswald-Richter KA, et al. Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-γ expression. J Clin Immunol. 2013;33:446–455. doi: 10.1007/s10875-012-9817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald-Richter KA, Beachboard DC, Zhan X, Gaskill CF, Abraham S, Jenkins C, Culver DA, Drake W. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res. 2010;11:161. doi: 10.1186/1465-9921-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ES, Wahlström J, Song Z, Willett MH, Wikén M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, et al. T cell responses to mycobacterial catalase–peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181:8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 33.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 34.Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol Cell Biochem. 2003;253:241–246. doi: 10.1023/a:1026020101379. [DOI] [PubMed] [Google Scholar]
- 35.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 37.Casagrande F, Bacqueville D, Pillaire MJ, Malecaze F, Manenti S, Breton-Douillon M, Darbon JM. G1 phase arrest by the phosphatidylinositol 3-kinase inhibitor LY 294002 is correlated to up-regulation of p27Kip1 and inhibition of G1 CDKs in choroidal melanoma cells. FEBS Lett. 1998;422:385–390. doi: 10.1016/s0014-5793(98)00043-x. [DOI] [PubMed] [Google Scholar]