Abstract
The extracellular matrix in asthmatic lungs contains abundant low-molecular-weight hyaluronan, and this is known to promote antigen presentation and allergic responses. Conversely, high-molecular-weight hyaluronan (HMW-HA), typical of uninflamed tissues, is known to suppress inflammation. We investigated whether HMW-HA can be adapted to promote tolerance to airway allergens. HMW-HA was thiolated to prevent its catabolism and was tethered to allergens via thiol linkages. This platform, which we call “XHA,” delivers antigenic payloads in the context of antiinflammatory costimulation. Allergen/XHA was administered intranasally to mice that had been sensitized previously to these allergens. XHA prevents allergic airway inflammation in mice sensitized previously to either ovalbumin or cockroach proteins. Allergen/XHA treatment reduced inflammatory cell counts, airway hyperresponsiveness, allergen-specific IgE, and T helper type 2 cell cytokine production in comparison with allergen alone. These effects were allergen specific and IL-10 dependent. They were durable for weeks after the last challenge, providing a substantial advantage over the current desensitization protocols. Mechanistically, XHA promoted CD44-dependent inhibition of nuclear factor-κB signaling, diminished dendritic cell maturation, and reduced the induction of allergen-specific CD4 T-helper responses. XHA and other potential strategies that target CD44 are promising alternatives for the treatment of asthma and allergic sinusitis.
Keywords: dendritic cell, hyaluronan, tolerance, allergy, T cell
Clinical Relevance
We describe a novel tolerogen that can be used to induce allergen-specific tolerance in animals sensitized previously to those allergens. These effects were durable for 2 weeks, providing an advantage over current desensitization protocols.
Hyaluronan (HA) is an extracellular matrix polysaccharide with well-established roles in pulmonary immunology and pathophysiology that are dependent, in part, on the length of HA polymers (1–4).
Intact, high-molecular-weight hyaluronan (HMW-HA) (>1 × 106 Da) predominates in uninjured or healing tissues (5) and is antiinflammatory in a variety of in vitro and in vivo model systems (6–11). HMW-HA prevents cell growth and differentiation (12), diminishes the production of inflammatory cytokines (13), and impairs phagocytosis (14). Many of these antiinflammatory effects of HMW-HA are mediated via CD44 (15).
Conversely, fragments of low-molecular-weight hyaluronan (LMW-HA), generated through catabolism of HMW-HA (16), predominate in chronically inflamed tissues and are typically proinflammatory (17–19). LMW-HA promotes the activation and maturation of dendritic cells (DC) (20), drives the release of proinflammatory cytokines (21), drives chemokine expression and cell trafficking (18), and promotes proliferation (22). Many of these proinflammatory effects are attributed to interactions with Toll-like receptor (TLR) 2 or TLR4 (22–24). LMW-HA promotes TLR-mediated nuclear translocation of nuclear factor-κB (NF-κB) (17). LMW-HA plays well-established roles in asthma (4, 25–27) and is associated with worse airway disease (11, 28–30). In light of these associations, HA size has been termed a natural biosensor for the state of tissue integrity (31).
Given the antiinflammatory properties of HMW-HA, we hypothesized that it could be used to promote tolerance to airway allergens. Specifically, we asked whether delivery of an allergen, bundled with HMW-HA, could ameliorate disease in a mouse model of allergic airway inflammation.
Materials and Methods
Reagents
HMW-HA (1.5 × 106 Da) was purchased from Genzyme (Cambridge, MA). XHA was generated using thiol-labeled HA (5 × 105 Da) sold as Glycosil by BioTime, Inc. (Alameda, CA). This was mixed together with polyethylene (glycol) diacrylate (PEGDA) crosslinker sold by BioTime Inc. at ratios per the manufacturer’s instructions. Phosphate-buffered saline (PBS) was used as the diluent before the addition of PEGDA to achieve a 0.1% hydrogel. This material was made fresh at the beginning of each 5-day intranasal (IN) administration period and was allowed to polymerize fully overnight before use. LMW-HA was generated from these reagents either via overnight digestion with hyaluronidase (HA’ase), as described previously (32) or via sonication for 60 minutes.
Transgenic Mice
All animals used, including CD44−/− mice (33), were on a BalbC background, with the exception of the IL-10−/− mice, which were on a C57Bl6 background. The animals were bred and maintained at a specific pathogen-free Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility at the Benaroya Research Institute. All experiments were approved by the Benaroya Research Institute Institutional Animal Care and Use Committees (protocol approval number 10116).
Allergic Airway Inflammation Protocol
Mice were primed intraperitoneally on Days 1 and 8 with 200 μL containing 100 μL alum (InvivoGen Alhydrogel 2%), 100 μL PBS, with 50 μg chicken ovalbumin (OVA) (Sigma A7642–1VL, St. Louis, MO). IN challenge reagents were prepared by mixing in the following order: (1) OVA, (2) PBS, (3) HA when used, and (4) PEGDA crosslinker when generating XHA. Challenge reagents sat at room temperature for at least 1 hour to ensure complete crosslinking of HA. XHA and controls were used at a final concentration of 0.1% wt/vol. OVA was used at 25 μL/ml. The mice were IN challenged for 5 consecutive days (Days 21–25), with 50 μL/day of challenge reagent after brief anesthesia with isoflurane. On the day after the last challenge (Day 26), the mice were killed with 2-2-2-Tribromethanol for tissue harvest. Bronchoalveolar lavages (BAL) were performed with 4 × 1 ml flushes of the lung with PBS. Before cell counting and flow cytometry, cells were lysed with ACK RBC lysis buffer (5′ 37°C, washed with media or PBS).
Physiologic Assessment of Airway Hyperreactivity
The mice were anesthetized with pentobarbital (90 mg/kg, i.p.) and intubated with a 20-gauge metal cannula via tracheotomy. Intubated mice were connected to a computer-controlled mechanical ventilator (flexiVent; SCIREQ, Montreal, QC, Canada) with a tidal volume of 10 ml/kg, rate of 180 bpm, and end-expiratory pressure of 3 cm H2O. After induction of neuromuscular paralysis with pancuronium (0.8 mg/kg), a slow inflation to total lung capacity as defined by an end-inspiratory pressure of 30 cm H2O was repeated twice. Lung resistance and elastance were then measured, using a single-frequency forced oscillation maneuver as described previously (34) at baseline and after progressive aerosolized methacholine challenge with concentrations ranging from 0 to 25 mg/ml.
Statistical Analyses
Unless otherwise stated, each experiment was performed at least two times. Data were analyzed using Student’s t test. Standard error is shown. All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Statistical significance was considered to be P < 0.05.
Results
Thiolation and Crosslinking Prevents the Catabolism of HMW-HA
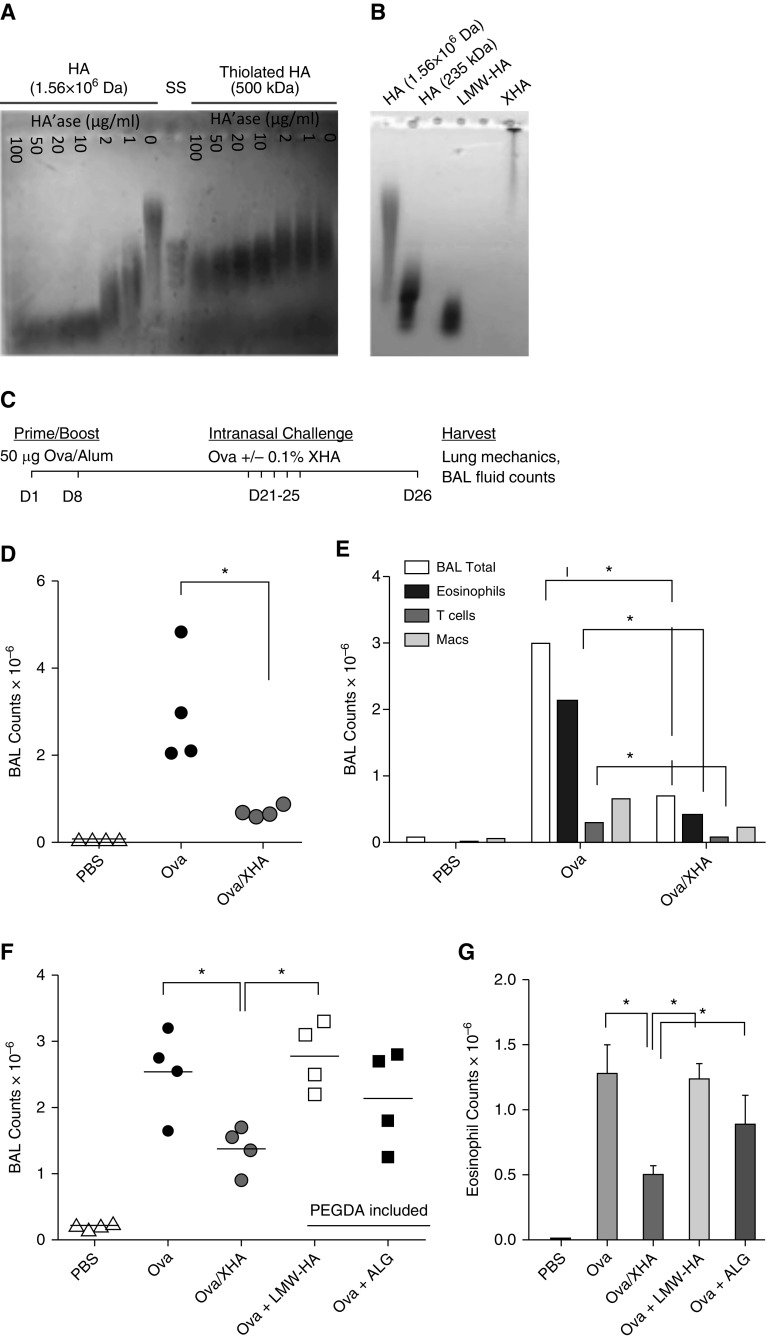
A practical concern with using HMW-HA to promote immune tolerance is the rapid catabolism of HMW-HA in vivo by HA’ases (16). We found that this can be overcome via the thiolation of the aldehyde groups on HA disaccharides. Although unadorned HMW-HA was broken down by increasing concentrations of HA’ase, HA that had been thiolated at ∼40% of its residues was highly resistant to enzymatic degradation (Figure 1A).
Figure 1.
Chemically modified high-molecular-weight hyaluronan (HMW-HA) (XHA) coupled with ovalbumin (OVA) (OVA/XHA) prevents allergic airway inflammation in mice previously sensitized to OVA. (A) Agarose gel electrophoresis of unmodified 1.56 × 106 443 Da HMW-HA and thiolated, 500 kD HMW-HA treated with increasing concentrations of hyaluronidase (HA’ase). A size standard (SS) ladder of 400–100 kD hyaluronan (HA) is also shown. (B) Agarose gel electrophoresis of unmodified 1.56 × 106 445 Da HMW-HA thiolated, 500 kD HMW-HA ± polyethylene (glycol) diacrylate (PEGDA) crosslinker (XHA) and controls. XHA was crosslinked overnight. All reagents were purchased commercially, and the aforementioned HA size characteristics are per the manufacturers’ information. Data in A and B are each representative of three experiments. (C) Schematic of the OVA-induced allergic airway inflammation protocol. (D) Total cell counts and (E) leukocyte subsets in bronchoalveolar lavage (BAL) fluid of mice rechallenged with OVA/XHA. Data in D and E are representative of eight independent experiments. Total cell counts (F) and eosinophils (G) in BAL fluid of mice treated with OVA, OVA/XHA, low-molecular-weight hyaluronan (LMW-HA), or alginate (ALG). *P < 0.05 by Student’s t test. SE is shown. Macs, macrophages.
To further enhance the stability of thiolated HA polymers, we crosslinked them using PEGDA. Although thiol groups will crosslink spontaneously over time through interactions with ambient oxygen, the addition of PEGDA allowed us to standardize the level of crosslinking. We called this crosslinked preparation “XHA.”
Chemical crosslinking also increased the effective size of HA. Compared with uncrosslinked HA and terminally digested LMW-HA fragments, XHA barely migrated through the gel (Figure 1B). This is consistent with XHA being crosslinked into a network of polymers. There were no lower bands, suggesting that the whole XHA preparation was involved in this crosslinking. A solution of 0.1% XHA could be readily administered IN, whereas more concentrated preparations were judged to be too viscous. Unless otherwise noted, the concentration of XHA used for all experiments was always 0.1%.
XHA Inhibits Allergic Airway Inflammation in Previously Sensitized Mice
We investigated the role of XHA in preventing allergic response in an OVA-induced mouse model of allergic airway inflammation (35). In this model, mice were sensitized by intraperitoneal (IP) injection of OVA with alum before OVA challenge on Days 21 through 25. On Day 26, the mice were killed and the development of allergic airway inflammation was evaluated. A schematic of this protocol is shown in Figure 1C.
Pretreatment with XHA on Days 16–20 did not protect the mice from allergic airway inflammation after OVA challenge on Days 21–25 (data not shown). Injection of XHA intraperitoneally, unlike OVA, which was given IN on Days 21–25, also did not protect mice from allergic airway inflammation (data not shown). These data indicated that XHA given at different times or via different routes than those of allergen did not prevent allergic airway inflammation.
We then evaluated the strategy of conjugating the OVA to XHA and delivering these molecules at the same time. Because OVA has four free thiol groups, the preincubation of OVA and thiolated HA can be expected to result in a covalently linked complex, particularly on addition of PEGDA. Using this preparation, the mice were challenged IN with OVA or OVA conjugated to XHA (which we call OVA/XHA) on Days 21 through 25 (Figure 1C). We found that the administration of OVA/XHA prevented airway inflammation in previously sensitized animals (Figure 1D). In more three independent experiments, each involving between four and eight mice per experimental group, this treatment typically led to an approximately threefold decrease in total BAL cell counts. We also observed decreased numbers of T cells (CD3+CD4+) and eosinophils (CD45+CD11c−/Siglec F+) (Figure 1E), key mediators of allergic disease.
HA Fragments and Unmodified HA Do Not Suppress Allergic Airway Inflammation
We sought to define the properties of XHA that are critical to suppressing allergic airway inflammation.
To investigate the role of HA polymer length, we evaluated LMW-HA in our model. This material was generated by sonication of the same HMW-HA used to make XHA and was identical in chemical composition. Because this LMW-HA was thiolated and crosslinker was added, it was also expected to covalently bind to OVA. However, it did not reduce total BAL counts or eosinophilia (Figures 1F and IG).
As a control for HA composition, we evaluated alginate (ALG), a polymer with charge and size properties similar to those of HMW-HA. It did not reduce total BAL counts or eosinophilia (Figures 1F and IG). The same held true for collagen (data not shown).
We then questioned whether chemical modification of HA is important. Chemically unmodified (nonthiolated) HMW-HA did not significant reduce BAL fluid counts (see Figure E1A in the online supplement). Conversely, thiolated HA without inclusion of the PEGDA crosslinker was sufficient to prevent allergic airway inflammation in our model (Figure E1B). However, thiolated HA can be expected to crosslink spontaneously on reaction with ambient oxygen, making the relative importance of thiolation versus crosslinking difficult to ascertain. Therefore, to standardize this crosslinking, we consistently added PEDGA to our XHA preparations.
Together, these data indicate that thiolation and perhaps crosslinking are necessary for the effectiveness of XHA.
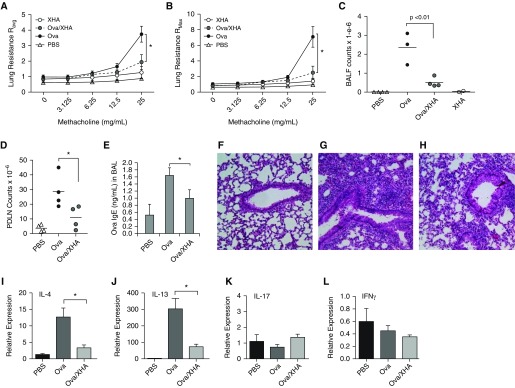
XHA Reduces Physiologic, Molecular, and Histologic Measurements of Allergic Airway Inflammation
Together with reduced cellular readouts of inflammation (decreased BAL fluid total counts and eosinophilia), OVA/XHA treatment reduced airway hyperresponsiveness as measured by change in lung resistance to increasing doses of methacholine challenge (Figures 2A–2C), pulmonary draining lymph node (PDLN) cell counts (Figure 2D), and OVA-specific IgE (Figure 2E). Histological analysis of lung sections likewise confirmed a decrease in cellular recruitment in mice treated with OVA/XHA (Figures 2F–2H). In addition, OVA/XHA-treated animals exhibited a decreased expression of Th2 cytokine mRNA (IL-4 and IL-13), but not the Th1 cytokine IFN-γ or the Th17 cytokine IL-17, in lung tissue (Figures 2I–2L).
Figure 2.
OVA/XHA prevents physiologic, histologic, and immunologic manifestations of allergic airway inflammation in mice sensitized previously to OVA. Average (A) and maximal (B) lung resistance in response to methacholine challenge. (C) Total BAL fluid (BALF) cell counts for the same mice as in A and B. (D) Pulmonary draining lymph node (PDLN) cell numbers for mice sensitized with OVA and then subsequently challenged with OVA or OVA/XHA. (E) OVA-specific IgE levels in BALF isolated from sensitized mice challenged with OVA or OVA/XHA. Data are for BAL pooled for five mice per group and measured in triplicate. Representative hematoxylin and eosin–stained lung sections for mice treated with (F) PBS, (G) OVA, or (H) OVA/XHA. Cytokine mRNA expression in pulmonary tissue of mice treated with OVA or OVA/XHA, specifically (I) IL-4, (J) IL-13, (K) IL-17, and (L) IFN-γ, all normalized to 18S expression. Tissues from five animals were pooled for this experiment and measured in triplicate. *P < 0.05 by a Student’s t test. SE is shown.
Together, these physiologic, histologic, molecular, and immunologic data strongly support the conclusion that OVA/XHA inhibits allergic airway inflammation in response to airway allergens.
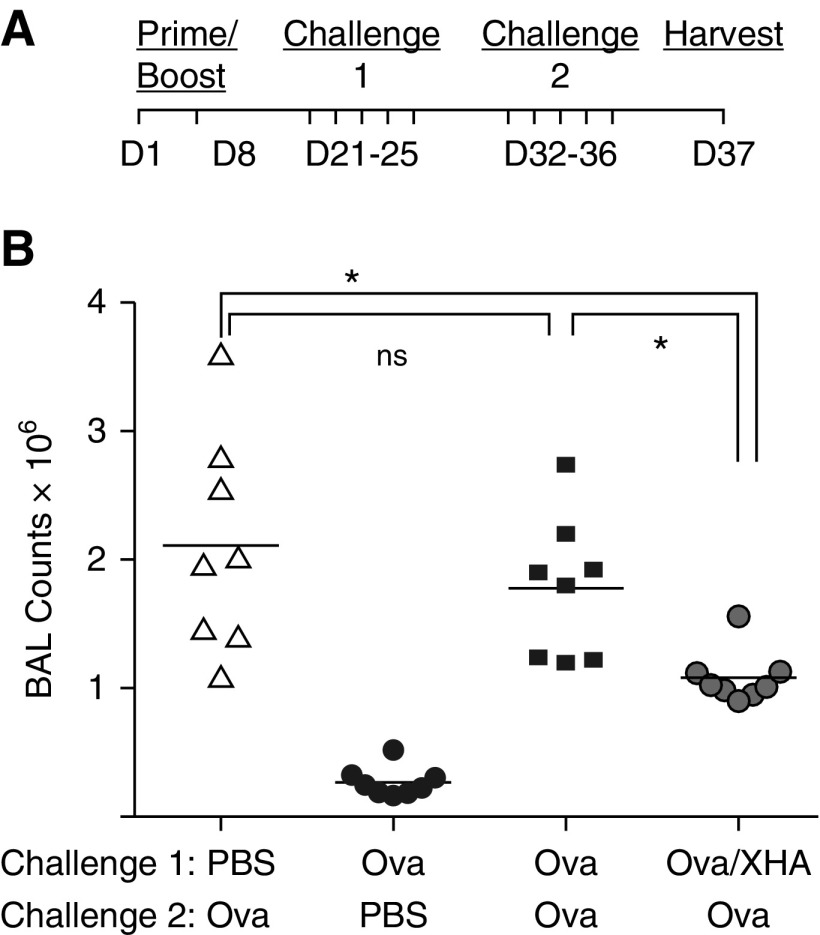
Antiinflammatory Effects of OVA/XHA on Allergic Airway Inflammation Are Durable for 2 Weeks
We also investigated if these OVA/XHA-mediated effects were durable over time. Animals were sensitized to OVA in albumin on Days 1 and 8 and were then challenged intranasally with OVA/XHA daily for challenge 1 (Days 21–25). These same animals were then rechallenged with OVA alone for challenge 2 (Days 32–36). BAL fluid cell counts were then assessed on Day 37. A schematic of this protocol is shown in Figure 3A. We found that OVA/XHA-exposed mice were protected from allergic airway inflammation in response to OVA, despite having last received OVA/XHA 12–16 days earlier (Figure 3B). This indicates that OVA/XHA-induced immune tolerance is durable in this model.
Figure 3.
Antiinflammatory effects of XHA on allergic airway inflammation are durable for 2 weeks. (A) Schematic of the protocol used to evaluate the durability of OVA/XHA-mediated tolerization. (B) BALF cell counts in response to challenge with OVA alone, in mice that received OVA/XHA 12–16 days earlier. Data are representative of three independent experiments. *P < 0.05 by Student’s t test. SE is shown. ns, not significant.
When we extended the time period between challenge 1 and challenge 2, so that the animals last received OVA/XHA 22–26 days earlier, the mice were no longer protected from OVA-induced allergic airway inflammation (Figure E2). This indicates that OVA/XHA-mediated suppression of allergic airway inflammation is durable but nonpermanent.
OVA/XHA Treatment Inhibits Local Allergen-Specific Responses
We also asked whether XHA could prevent allergic airway inflammation in response to a different allergen, cockroach antigen (CRA). Using a protocol using IN sensitization with CRA (36), we found that CRA/XHA treatment reduced cellular recruitment significantly in the PDLN (Figure E3A), but this effect did not reach statistical significance in the BAL (Figure E3B). This distinction may reflect the fact that inflammation in the CRA model is less pronounced in the BAL, relative to the OVA model.
We also investigated whether treatment with XHA and one allergen could induce tolerance to another allergen using the protocol in Figure E3C. When mice sensitized to OVA (on Days 1 and 8) subsequently received CRA/XHA (challenge 1; Days 21–25), they nonetheless developed robust inflammation in response to subsequent exposure to OVA (challenge 2; Days 32–36) (Figure E3D). This suggests that XHA does not induce tolerance to allergens with which it is not coadministered.
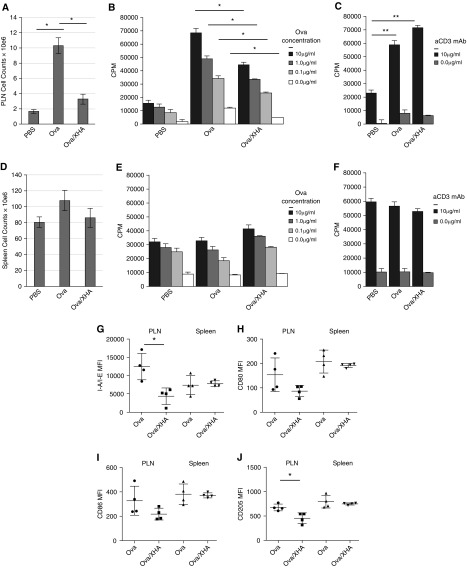
We then asked whether OVA/XHA treatment results in diminished allergen-specific responses ex vivo. OVA-specific responses were diminished ex vivo in PDLN cells isolated from animals treated previously with OVA/XHA (Figures 4A and 4B), whereas polyclonal responses to anti-CD3/28 antibodies were not reduced (Figure 4C). In contrast, splenocyte counts were not diminished by OVA/XHA treatment (Figure 4D), and splenocytes isolated from mice that received either OVA or OVA/XHA had comparable proliferation in response to both OVA, as well as to anti-CD3/28 Ab ex vivo (Figures 4E and 4F).
Figure 4.
XHA treatment inhibits proliferative responses to allergen but not to polyclonal stimulus. (A–C) PDLN lymphocytes and (D–F) splenocytes were isolated from animals sensitized to OVA and then challenged with PBS, OVA, or OVA/XHA. Cell counts in (A) PDLN and (D) spleens from mice treated with PBS or OVA or OVA/XHA are shown. Lymphocytes and splenocytes were then stimulated ex vivo with either (B and E) OVA or (C and F) anti-CD3 at the concentrations indicated for 72 hours. Tritiated thymidine was added for the final 24 hours. Data are representative of two independent experiments. In a separate experiment, PDLN lymphocytes and splenocytes were stained directly ex vivo for (G) I-A/I-E, (H) CD80, (I) CD86, and (J) CD205. MFIs were calculated relative to antibody-appropriate isotype controls. *P < 0.05; **P < 0.01. Data are representative of two experiments. CPM, counts per minute; MFI, median fluorescence intensity; PLN, pulmonary lymph node.
These data indicate that local, antigen-specific immune responses are inhibited after OVA/XHA treatment of mice sensitized previously to OVA. Furthermore, it is notable that polyclonal, systemic responses were not reduced, indicating that OVA/XHA treatment does not lead to generalized immune suppression.
One possible explanation for our results is that formulation of antigen with XHA leads to sequestering of antigen and a lower effective delivery of the antigen. To evaluate this, we repeated our in vivo assay using Dye quenched Ovalbumin (DQ OVA) conjugated to the pH-insensitive BODIPY (4,4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene) dye, which fluoresces green on proteolytic degradation of DQ OVA. Using this tool, we observed that coupling OVA to XHA did not affect antigen uptake and processing by lung resident antigen-presenting cell subsets (including CD11b+ CD11c+ DC and CD11c+ CD103+ DC) (Figure E4). The gating strategy for these cells is shown in Figure E5 and their absolute numbers are shown in Figure E6. Similarly, we observed that coupling OVA to XHA did not affect the fraction of DQ OVA+ DC present in the PDLN (Figures E7 and E8). These data indicate that formulation of OVA/XHA does not lead to immune ignorance via a lower effective delivery of antigen.
OVA/XHA Effects on Immune Tolerance Are IL-10 Dependent
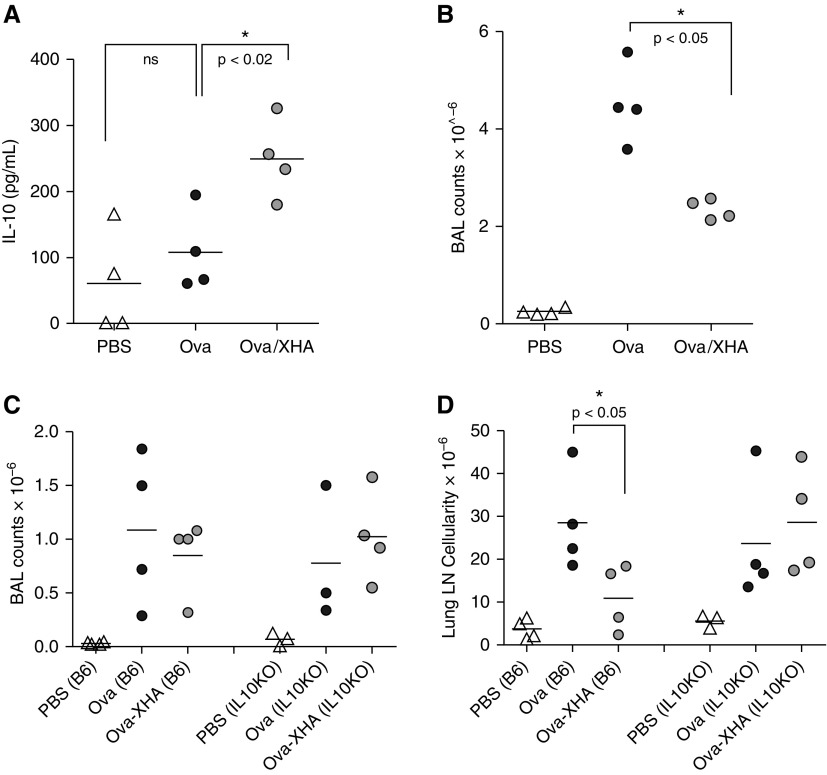
To investigate how our findings are consistent with established mechanisms of tolerance, we evaluated whether OVA/XHA treatment promotes IL-10 production. We specifically examined IL-10 because it is known to contribute to tolerance to airway allergens (37) and because, as we reported previously, HMW-HA promotes IL-10 production by multiple cell types in vitro (10, 38). We indeed saw that OVA/XHA treatment promotes IL-10 production (Figures 5A and 5B). Moreover, the tolerizing effect of XHA was lost in IL-10–deficient mice (Figures 5C and 5D). From these data, we concluded that the mechanism of OVA/XHA-mediated tolerance is IL-10 dependent.
Figure 5.
OVA/XHA effects are IL-10 dependent. Mice sensitized previously to OVA were administered intranasal OVA or OVA/XHA. Cells were isolated from total BAL fluid via centrifugation (4 × 1 ml flushes of the lung with PBS) and were activated in vitro with anti-CD3 for 48 hours. (A) IL-10 levels in cell culture supernatants as measured by ELISA. (B) BAL fluid counts for the same animals as in A. The same protocol was repeated in IL-10−/− mice, and total cell counts were measured in (C) BAL fluid and (D) PLN. *P < 0.05. LN, lymph node.
XHA Prevents DC Maturation In Vivo
The data in Figure 4 suggest a role for antigen presentation in XHA-mediated inhibition of allergic airway inflammation responses. Consistent with this, CD11c+ DC in PDLN from OVA/XHA-treated mice had reduced expression of the cell-surface markers involved in antigen presentation and costimulation, including I-A/I-E (MHC-II) and CD205, compared with mice treated with OVA alone. However, the effects on the costimulatory markers CD80 and CD86 were not statistically significant. These effects were observed in CD11c+ DC from PDLN but not the spleen (Figures 4G–4J). These particular cell surface markers were examined because of their role in antigen presentation and costimulation.
Similar results were seen in CD11c+ DC in BAL fluid. CD11c+ DC isolated from mice treated with OVA/XHA had elevated expression of CD62L (Figures E9A andE9B) (a marker of immature DC) and reduced levels of I-A/I-E, CD80, and CD86 compared with similar cells from animals treated with OVA alone (Figures E9C to E9H). Together with the PDLN data above, these results are indicative of decreased local DC maturation in vivo on OVA/XHA treatment.
We considered whether OVA/XHA treatment might lead to altered recruitment of specific DC subsets known to be involved in allergic asthma. X OVA/HA treatment was associated with an increase in the fraction of CD11b+CD11c+ cells in the BAL fluid of previously sensitized animals (Figures E10A–E10D). These cells were both I-A/I-E+ and LY6C+ (Figures E10E and E10F), a profile consistent with recently recruited LY6C+ monocytes, a cell subset capable of driving inflammation in a number of different settings (39–41). However, the absolute numbers of these CD11b+CD11c+ cells were not increased on OVA/XHA treatment (Figure E6), indicating that most of their increase reflects reduced numbers of other cell populations involved in allergic airway inflammation responses. We did not observe heightened numbers of CD11c+ CD103+ DC or CD3−CD19−CD11c+mPDCA plasmacytoid DC (Figures E10G and E10H). These data suggest that OVA/XHA treatment does not meaningfully alter the representation of these DC populations.
XHA Prevents DC Maturation In Vitro
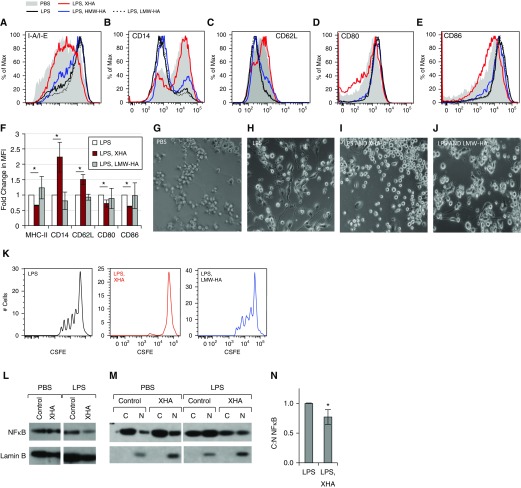
We next evaluated whether XHA could influence the in vitro maturation of bone marrow–derived DC (BMDC). Immature BMDC were cultured on plates coated with 1% XHA and controls and were treated with LPS to promote their maturation. In this setting, XHA treatment of BMDC prevented up-regulation of I-A/I-E, CD80, and CD86 and down-regulation of CD14 and CD62L, resulting in retention of an immature DC phenotype, compared with those treated with LPS alone. Culturing cells on plates coated with HMW-HA had a more modest effect, whereas soluble LMW-HA actually promoted DC maturation, as has been reported previously (17, 19) (Figures 6A–6F). These studies were performed with plate-bound 1% XHA to facilitate direct contact between this material and the cells. Analogous but less potent effects were seen with 1% soluble XHA (Figure E11). This inhibition of maturation was also evident at the level of cell morphology. LPS and LMW-HA promoted DC spreading and development of dendrites, whereas culturing cells in the setting of a coating of 1% XHA prevented this (Figures 6G–6J). ALG did not inhibit antigen presentation (Figure E12). Consistent with these phenotypic profiles, plate-bound 1% XHA-treated DC were less efficient at antigen presentation as assessed by proliferation of T cells isolated from animals immunized previously with OVA and labeled with carboxyfluorescein succinimidyl ester (CFSE) (Figure 6K).
Figure 6.
XHA prevents bone marrow–derived dendritic cell (BMDC) maturation in response to LPS. BMDC were treated with LPS for 24 hours in the setting of a 1% coating of XHA or soluble LMW-HA. Representative histograms showing the change in cell surface markers including (A) I-A/I-E, (B) CD14, (C) CD62L (D) CD80, and (E) CD86 after treatment with XHA and controls. (F) Fold change in MFI for the same markers as in A–E. This figure includes data from three independent experiments. Representative appearance of BMDC cultured in the setting of (G) PBS, (H) LPS, (I) LPS and XHA, or (J) LPS and LMW-HA. (K) Proliferation in response to OVA, as measured by carboxyfluorescein succinimidyl ester (CFSE) dye dilution, for CD4+T491 cells isolated from animals immunized previously with OVA and cultured for 72 hours with BMDC cultured previously for 24 hours with LPS ± XHA or LMW-HA. Western blot for the p65 subunit of nuclear factor-κB (NF-κB) for (L) whole cell lysates or (M) the corresponding cytosolic (C) and nuclear (N) fractions of dendritic cells, treated with PBS or LPS ± XHA. (N) Ratios of C to N NF-κB in lysates for three independent experiments. *P < 0.05 by Student’s t test. SE is shown.
XHA Inhibits NF-κB Nuclear Translocation
Given that LPS is known to drive DC maturation through NF-κB–dependent mechanisms, we questioned whether plate-bound 1% XHA inhibits NF-κB signaling in BMDC treated with LPS. We assessed this by examining translocation of the p65 subunit of active NF-κB from the cytosol to the nucleus with or without LPS treatment for 24 hours. We observed a relatively constant level of total amount of NF-κB, as measured by the ratio between NF-κB and the nuclear protein lamin B1 (Figure 6L). However, plate-bound 1% XHA inhibited nuclear translocation of NF-κB instigated by LPS treatment (Figure 6M). This increased the C:N ratio, as shown for five independent experiments (Figure 6N). Together, these data suggest that plate-bound 1% XHA inhibits DC maturation and NF-κB signaling in response to proinflammatory signals.
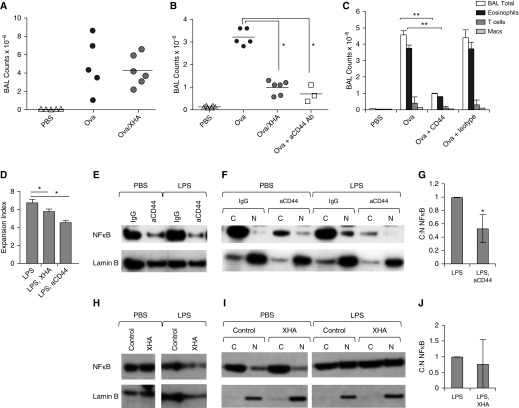
XHA Effects Are CD44 Dependent
To evaluate whether this OVA/XHA-mediated inhibition of allergic airway inflammation was dependent on CD44, the HA receptor, we repeated these studies in CD44−/− mice. Strain-matched BalbC.CD44−/− mice had robust airway inflammation despite treatment with OVA/XHA (Figure 7A), indicating that CD44 is required for XHA-mediated inhibition of airway inflammation. Administration of anti-CD44 Ab IN to wild-type BalbC mice together with OVA during the rechallenge stage decreased total BAL cells (Figure 7B) and eosinophilia (Figure 7C), suggesting that this treatment had effects analogous to those of XHA. In vitro treatment with plate-bound CD44 antibodies (which might be expected to crosslink CD44 in a manner analogous to that of HMW-HA [42, 43]) likewise limited the LPS-mediated effects on DC antigen presentation, as assessed by proliferation of CFSE-labeled T cells (Figure 7D). Consistent with this, plate-bound CD44 antibodies also inhibited nuclear translocation of NF-κB instigated by LPS treatment (Figures 7E–7G). NF-κB nuclear translocation induced by stimuli other than LPS, including Pam3Csk4 and tumor necrosis factor-α, were also inhibited by aCD44 Ab (Figure E13). These data indicate that XHA inhibits NF-κB signaling via a CD44-dependent pathway, consistent with established roles for CD44 in the negative regulation of TLR signaling (44–46).
Figure 7.
XHA-mediated inhibition of allergic airway inflammation is CD44 dependent. (A) BAL cell counts for CD44−/− mice after rechallenge with OVA or OVA/XHA. (B) BAL cell counts for wild-type (CD44+/+) mice treated with OVA, OVA/XHA, or anti-CD44 antibody (Ab). (C) BAL cell counts for mice treated as in B but now for different leukocyte subsets. *P < 0.05. Data in A–C are representative of three experiments. (D) Proliferation in response to OVA, as measured by CFSE dye dilution, for CD4+T cells isolated from animals immunized with OVA and cultured for 72 hours with BMDC cultured previously for 24 hours with LPS ± XHA or anti-CD44 Ab. Western blots for the p65 subunit of NF-κB generated using (E) whole cell lysates or (F) the corresponding C and N fractions of dendritic cells treated with PBS or LPS ± aCD44 Ab or an isotype-matched control IgG. (G) Ratios of C to N NF-κB in lysates of dendritic cells treated as in F, now for three independent experiments. (H–J) Similar data as shown in Figures 6L–6N only now generated using cells from CD44−/− mice. *P < 0.05; **P < 0.01 by Student’s t test. SE is shown.
To evaluate whether XHA-mediated inhibition of NF-κB nuclear translocation was dependent on CD44, the HA receptor, we repeated the assay in Figures 6L–6N now using BMDC derived from CD44−/− mice. We observed that, in the absence of CD44, plate-bound 1% XHA had a nonsignificant impact on NF-κB signaling (Figures 7H–7J).
Together, these data support a model whereby OVA/XHA prevents DC maturation in a CD44-dependent manner, thereby limiting efficient presentation of allergens to previously activated, allergen-specific cells.
Discussion
We have discovered that HMW-HA, a polymer characteristic of healthy lung tissue, promotes tolerance to airway allergens in an IL-10–dependent manner. This effect required chemical stabilization of HA to prevent its catabolism. This thiolated and crosslinked preparation, which we call XHA, improved the physiologic (responses to methacholine challenge), histologic (airway infiltrates), and immunologic (cytokine levels and BAL fluid counts) aspects of allergic airway inflammation. These effects included elements of both the acute response (e.g., OVA-specific IgE levels) and late-phase responses (e.g., eosinophil counts) in mice sensitized previously to allergens. These data suggest that the antiinflammatory cues that intact HMW-HA provides can be harnessed to treat hypersensitivity to airway allergens in this model.
Although our data are consistent with the generally antiinflammatory properties of HMW-HA, the chemical modifications that produced XHA were necessary for its effectiveness in our models. Thiolation enhances stability against HA’ases and, together with crosslinking, may prevent rapid catabolism of XHA. Crosslinking transforms HA into networks of interlinked polymers of increased effective size and may also contribute to the stability of XHA in vivo.
One possible explanation for our results is that, because of lower effective delivery of antigen to the tissue, the formulation of antigen with XHA leads to immune ignorance, rather than immune tolerance. Several lines of evidence argue against this interpretation. First, we demonstrated that coupling OVA to XHA does not affect antigen uptake and processing by lung resident antigen-presenting cells, nor their migration into the draining lymph nodes. Second, we observed effects using allergens (OVA and CRA) with different physical properties, making a specific masking effect unlikely. Third, tolerizing effects were not seen using LMW-HA, a material with chemical composition identical to that of XHA. Fourth, tolerance was not seen in the setting of ALG, a polymer with properties similar to those of HA that does not interact with CD44, the major cell-surface HA-binding transmembrane glycoprotein (47). Fifth and finally, XHA was not efficacious in CD44−/− mice, and this would not be expected to be the case in the setting of immune ignorance. Together, these lines of evidence refute the argument that immune ignorance is responsible for the effects observed here.
Our data indicate that XHA promotes an immature DC phenotype and thereby prevents efficient presentation of allergens to previously activated, allergen-specific cells. Indeed, XHA treatment trumps the proinflammatory effects of LPS on DC maturation and NF-κB signaling. These data are perhaps consistent with roles for immature DC in the induction of IL-10–based immune tolerance (48). Our data do not, however, rule out XHA effects on other cell types present in the inflamed lung.
The effects of OVA/XHA are CD44 dependent. This is consistent with reports that CD44 signaling inhibits NF-κB DNA activation (44, 45, 49) and also with reports that both MyD88 expression levels and nuclear translocation of NF-κB are increased in CD44−/− mice (44, 45). We propose that XHA may be a biomimetic of the naturally occurring complexes of HMW-HA and hyaladherins that provide what we have called “tissue integrity signals” that dampen inflammation in healing or uninjured tissues (15). To our knowledge, XHA is the first technology to use an endogenous tolerogen.
XHA may have therapeutic potential in allergic asthma. The current standard of care, desensitization, prevents allergic responses by the administration of daily treatments of allergens (50). The durability of XHA over time (∼2 wk) potentially offers a substantial advantage over these daily regimens. Because HMW-HA is a natural component of human tissues, it is highly biocompatible and would not be expected to promote toxicities (51) such as those associated with inhaled particulate matter (52). Our vision is that antigen/XHA solutions could be administered as an intranasal spray or rinse to treat allergic asthma and sinusitis or be adapted to enhance current desensitization protocols.
Conclusions
The finding that a simple, biocompatible modification (thiolation) protects HMW-HA from catabolism also has broad applicability. HMW-HA is Food and Drug Administration approved for many indications (53–55). The use of thiolated HA as a starting material may radically improve the efficacy of these products by dramatically increasing their longevity.
Acknowledgments
Acknowledgments
The authors thank J. Idoyaga and H. Ishak for their reading of this manuscript and their helpful comments. They also thank Tom Zarembinski at BioTime Inc. for his help in providing the reagents and his insight into BioTime products.
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01 DK096087-01, R01 HL113294-01A1, and U01 AI101984 (P.L.B.) and grant 5 T32 AI07290 (S.M.R.); by a Swiss National Science Foundation early postdoc mobility grant (K.Y.); by the Child Health Research Institute; by Stanford NIH-NCATS-CTSA grant UL1 TR001085 (K.Y.); Gabilan Stanford Graduate Fellowship for Science and Engineering and the Lubert Stryer Bio-X Stanford Interdisciplinary Graduate Fellowship (J.M.S.); and by NIH/NIAMS R01 AR064206 (K.M.).
Author Contributions: J.A.G., P.H., V.A.d.J.P., M.J.B., W.A.A., N.F., and P.L.B. designed the experiments; J.A.G., K.Y., S.M.R., P.M., P.H., B.A.F., J.M.S., H.H., G.K., C.M., and W.A.A. performed the experiments; K.M., S.F.Z., S.B., S.G.K., K.N., and W.A.A. contributed resources, reagents, and technologic expertise; and P.L.B. wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0111OC on September 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Laurent TC, Laurent UB, Fraser JR. The structure and function of hyaluronan: an overview. Immunol Cell Biol. 1996;74:A1–A7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 2.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauer ME, Majors AK, Comhair S, Ruple LM, Matuska B, Subramanian A, Farver C, Dworski R, Grandon D, Laskowski D, et al. Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J Biol Chem. 2015;290:23124–23134. doi: 10.1074/jbc.M115.663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 8.Huang P-M, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu Y-Y, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 2010;15:1131–1139. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 9.Galeano M, Polito F, Bitto A, Irrera N, Campo GM, Avenoso A, Calò M, Cascio Lo P, Minutoli L, Barone M, et al. Systemic administration of high-molecular weight hyaluronan stimulates wound healing in genetically diabetic mice. Biochim Biophys Acta. 2011;1812:752–759. doi: 10.1016/j.bbadis.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci USA. 2011;108:7938–7943. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW, Sr, Stober VP, Trempus CS, Garantziotis S, et al. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L891–L903. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann A, Schinzel R, Palm D, Riederer P, Münch G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-κB activation and cytokine expression. FEBS Lett. 1999;453:283–287. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- 14.Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435–446. [PMC free article] [PubMed] [Google Scholar]
- 15.Ruppert SM, Hawn TR, Arrigoni A, Wight TN, Bollyky PL. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol Res. 2014;58:186–192. doi: 10.1007/s12026-014-8495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-κB/I-κB alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 21.de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, Danese S, Fiocchi C, Stern R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am J Pathol. 2009;174:2254–2264. doi: 10.2353/ajpath.2009.080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 23.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 24.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med. 2010;181:666–675. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 25.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411.e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayars AG, Altman LC, Potter-Perigo S, Radford K, Wight TN, Nair P. Sputum hyaluronan and versican in severe eosinophilic asthma. Int Arch Allergy Immunol. 2013;161:65–73. doi: 10.1159/000343031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–134. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh S, Hoselton SA, Dorsam GP, Schuh JM. Hyaluronan fragments as mediators of inflammation in allergic pulmonary disease. Immunobiology. 2015;220:575–588. doi: 10.1016/j.imbio.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 30.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutás G, Bajnok E, Gál I, Finnegan A, Glant TT, Mikecz K. CD44-specific antibody treatment and CD44 deficiency exert distinct effects on leukocyte recruitment in experimental arthritis. Blood. 2008;112:4999–5006. doi: 10.1182/blood-2008-04-150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates JH. Pulmonary mechanics: a system identification perspective. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:170–172. doi: 10.1109/IEMBS.2009.5333302. [DOI] [PubMed] [Google Scholar]
- 35.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 36.Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, El Mays T, Gilmore BF, Walker B, Gordon JR, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186:3164–3172. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- 37.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124:4678–4680. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-β. J Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 42.Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MGEG, Moghimi SM, et al. Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release. 2011;156:231–238. doi: 10.1016/j.jconrel.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, Richter RP. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J Biol Chem. 2010;285:30170–30180. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 45.Kawana H, Karaki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, Harigaya K. CD44 suppresses TLR-mediated inflammation. J Immunol. 2008;180:4235–4245. doi: 10.4049/jimmunol.180.6.4235. [DOI] [PubMed] [Google Scholar]
- 46.Muto J, Yamasaki K, Taylor KR, Gallo RL. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009;47:449–456. doi: 10.1016/j.molimm.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Puré E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 48.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda T. Hyaluronan inhibits Akt, leading to nuclear factor-κB down-regulation in lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci. 2011;115:509–515. doi: 10.1254/jphs.10244fp. [DOI] [PubMed] [Google Scholar]
- 50.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 51.Knudsen KB, Northeved H, Ek PK, Permin A, Andresen TL, Larsen S, Wegener KM, Lam HR, Lykkesfeldt J. Differential toxicological response to positively and negatively charged nanoparticles in the rat brain. Nanotoxicology. 2014;8:764–774. doi: 10.3109/17435390.2013.829589. [DOI] [PubMed] [Google Scholar]
- 52.Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prestwich GD, Kuo J-W. Chemically-modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol. 2008;9:242–245. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- 54.Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 55.Prestwich GD, Erickson IE, Zarembinski TI, West M, Tew WP. The translational imperative: making cell therapy simple and effective. Acta Biomater. 2012;8:4200–4207. doi: 10.1016/j.actbio.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]