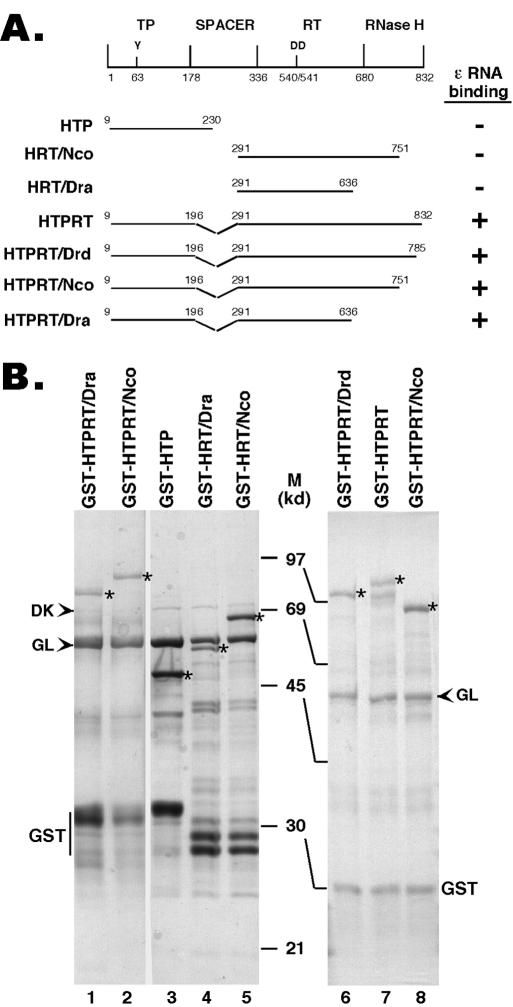
FIG. 1.
Expression of truncated HBV RT proteins and domains. (A) Schematic diagram of the HBV RT proteins and domains expressed. Shown on the top is the domain structure of the HBV RT, with the primer tyrosine (Y63) and the double aspartate (D540/D541) in the RT active site denoted. The ends of the truncations and deletions are indicated. The truncated HBV RT proteins and domains were expressed as GST fusion proteins in bacteria, purified by using glutathione resins, resolved by SDS-PAGE, and detected by Coomassie blue staining (B). The intact fusion proteins are indicated by asterisks. The two bacterial chaperone proteins, DnaK (DK) and GroEL (GL), copurifying with the RT proteins, are indicated by arrowheads. The major degradation product, GST, is also indicated. M, molecular mass in kDa; HTP, HBV terminal protein.