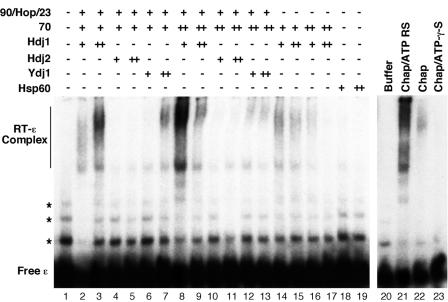
FIG. 4.
Reconstitution of HBV RT-ɛ interaction as detected by a gel-mobility shift assay. Purified GST-HTPRT-Drd (20 ng) was incubated with 32P-labeled HBV ɛ RNA, in the presence of the indicated chaperone (Chap) factors. The amount of chaperone factors used per reaction was as follows: Hsp90 (90), 360 ng; Hop, 375 ng; p23 (23), 100 ng; Hsp70 (70), 1 μg (+) or 3 μg (++); Hdj1 (or Hdj2 or Ydj1), 200 ng (+) or 600 ng (++); and Hsp60, 170 ng (+) or 510 ng (++). An ATP regenerating system was added in all reactions except in lane 22, where it was omitted, and lane 23, where it was replaced by the nonhydrolyzable ATP analog ATP-γ-S. In lanes 21 to 23, all five chaperone factors were added: Hsp90, 360 ng; Hop, 375 ng; p23, 100 ng; Hsp70, 3 μg; and Hdj1, 200 ng. After a 2-h incubation at 30°C, the reactions were resolved on a native polyacrylamide gel, which was then dried and subjected to autoradiography. The labeled free ɛ RNA and the RT-ɛ complex are indicated. The minor bands (marked by asterisks) above the marked free probe species represented minor structural isoforms of the free RNA, which were not always detectable (see also Fig. 5 to 7).