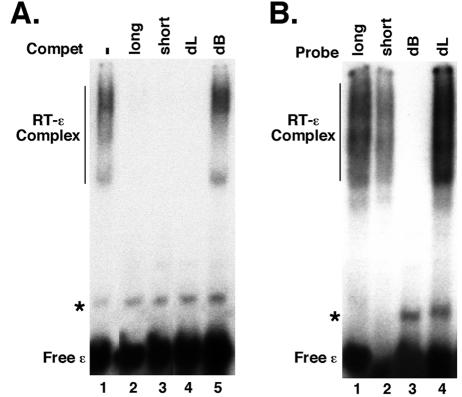
FIG. 7.
The internal bulge, but not the apical loop, of ɛ was required for RT binding. (A) GST-HTPRT/Drd (20 ng) was incubated with 32P-labeled HBV ɛ RNA, in the presence of five chaperone factors: Hsp90 (360 ng), Hsp70 (3 μg), Hop (275 ng), Hdj1 (200 ng), and p23 (100 ng). As indicated, the following competitors (in 100-fold excess) were added: unlabeled HBV ɛ (wild-type or long) (lane 2), a truncated HBV ɛ variant with a shorter lower stem (short) (lane 3), an HBV ɛ variant with its apical loop deleted (dL) (lane 4), or with its internal bulge deleted (dB) (lane 5). (B) The wild type and three different HBV ɛ variants were 32P-labeled and used in the RT binding assay. The labeled ɛ RNA and the RT-ɛ complex are indicated. The minor band (marked by an asterisk in each panel) represented a minor structural isoform of the free RNA.