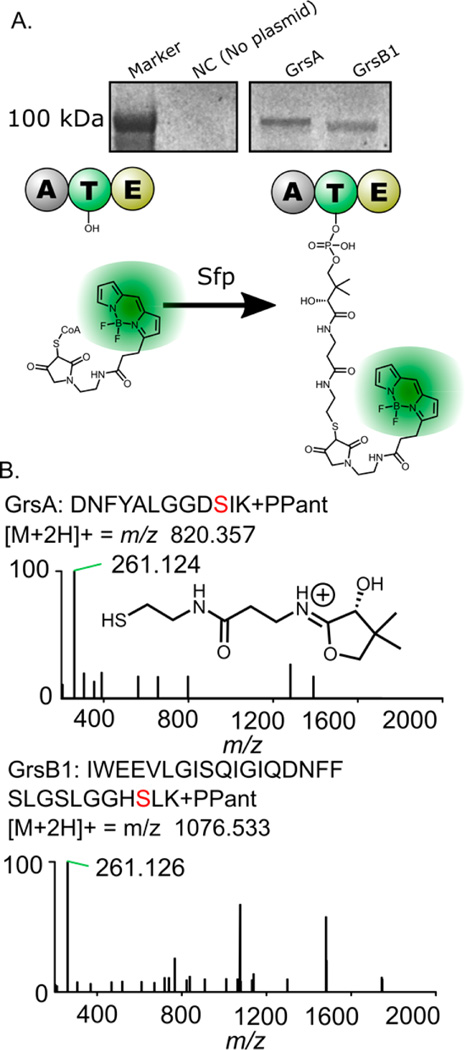
Figure 3.
Experiments showing that GrsA and GrsB1 are present in their active (holo) forms. Panel A shows the fluorescent labeling of GrsA and GrsB1 on the thiolation domain active sites with a conjugated Bodipy-CoA fluorophore (see Figure S3 for complete gel image). Panel B top shows the MS2 spectrum resulting from the fragmentation of a precursor peptide containing the GrsA phosphopantetheine modification. Panel B bottom shows the MS2 spectrum for the corresponding GrsB1 T-domain peptide, indicating the mass of the observed pantetheine-derived ion.