FIG. 1.
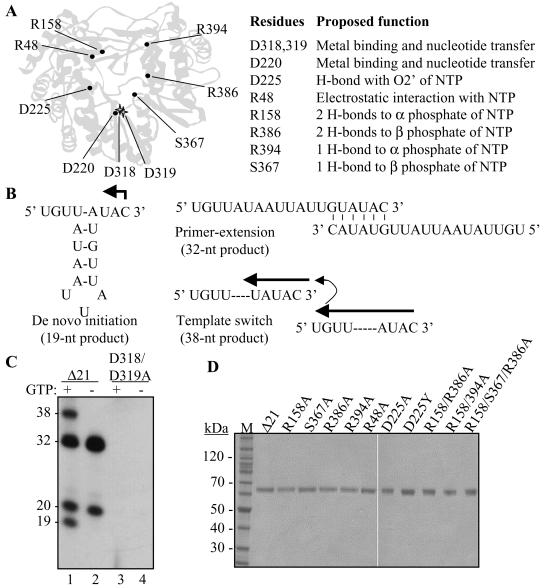
Mutations within the catalytic pocket of the HCV RdRp and assays for activities of the HCV RdRp. (A) Locations of the mutations in the HCV RdRp and their proposed function in interacting with the initiation GTP. The two stars representing D318, D319, and D220 denote the location of the site for nucleotide polymerization. (B) The modes of RNA synthesis that are directed by RNA LE19. A monomer of LE19 could direct de novo initiation from the 3′-most cytidylate, producing a 19-nt product. In the event that two LE19 molecules base pair with sequences at their 3′ ends, the resultant heterodimer could direct the synthesis of a 32-nt primer extension product which, based on the sequence, could be generated in the absence of GTP. Should the ternary complex translocate from one template to another without release of the nascent RNA, a process called template switch, a product of 38 nt is generated. (C) Demonstration of the products from LE19 template by the prototype HCV RdRp, Δ21, and one with mutations at the active site. GTP is needed for de novo initiation, resulting in 19-nt RNA and 38-nt template switch products generated after de novo initiation. GTP is not needed to produce a primer extension product of 32- and 20-nt nontemplated nucleotide addition to LE19. The sizes of the products have been previously characterized (22, 23, 25). (D) Preparation of recombinant proteins used in this study. The proteins were separated by sodium dodecyl sulfate-polyacrylamide electrophoresis (4 to 12% polyacrylamide) and were stained with Coomassie blue. The names of the mutant proteins are derived from the respective amino acid, the position along the NS5B polypeptide, and the final amino acid at that position.
