FIG. 5.
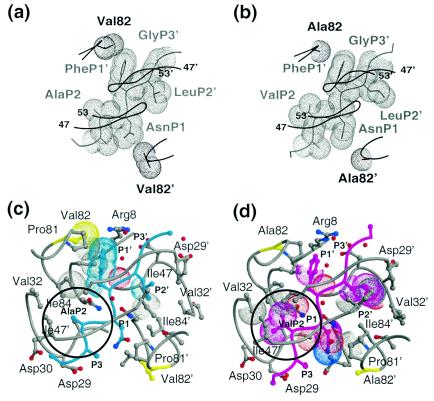
Variations in the substrate-protease van der Waals interactions between the WTNC-p1WT and AP2VNC-p1V82A complexes. van der Waals surfaces of protease residue 82, viewed down the dimer twofold axis, and neighboring atoms of the corresponding NC-p1 substrate: (a) WTNC-p1WT complex (note that Val82 contacts PheP1′) and (b) AP2VNC-p1V82A complex (note that no contact is made between Ala82 and the substrate). Substrates and proteases are distinguished in gray and black, respectively. A Cα trace of the overlying flaps is also shown. (c) Active site residues in the WTNC-p1WT. The protease is shown in gray and the substrate in cyan. Protease residues within 4.2Å of the substrate are shown in ball-and-stick representation, and residue 82 is highlighted in yellow. van der Waals contacts that are lost compared with the AP2VNC-p1V82A complex are highlighted with dotted surfaces and the P2 site is circled. (d) Active site residues in the AP2VNC-p1V82A. The protease is shown in gray, and the substrate is shown in magenta; protease residues within 4.2Å of the substrate are shown in ball-and-stick representation, and residue 82 is highlighted in yellow. van der Waals contacts that are lost compared with the WTNC-p1WT complex are highlighted with dotted surfaces, and the P2 site is circled.
