FIG. 4.
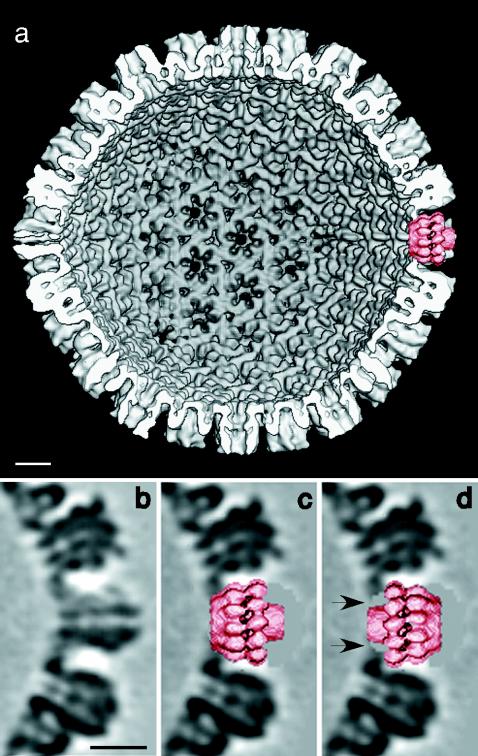
Fitting of the UL6 portal protein into a vacant vertex in the HSV-1 capsid. (a) Interior view of a capsid model at 18-Å resolution (4) from which a penton of VP5, the major capsid protein, was computationally excised and replaced by the portal (right-hand side at 3 o'clock). Panel b shows a section through a vertex site occupied by a VP5 pentamer and is compared in panels c and d with the same site with an implanted portal in both orientations. In one orientation (c), the portal fits snugly into this cavity. In the other orientation (d), the portal also fits but makes only tenuous contact with the surrounding VP5 molecules, leaving gaps between the molecules and the capsid (arrows), suggesting a less stable interaction. Bars, 100 Å.
