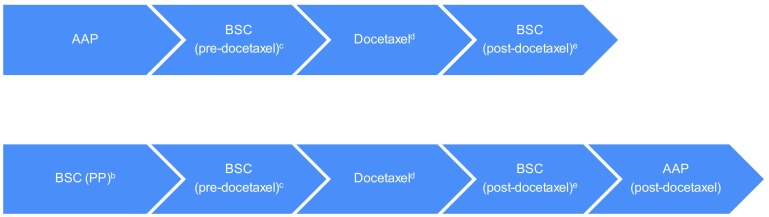
Fig. 1.
Visual representation of the DES model (see Figs. 5.1 and 5.2 from the ERG report [3] for more details). DES discrete event simulation, AAP abiraterone acetate in combination with prednisone/prednisolone, BSC best supportive care, PP placebo plus prednisone/prednisolone, ERG Evidence Review Group, ECOG Eastern Cooperative Oncology Group, PS performance score. a Patients could die in all stages of the model, except during AAP, BSC (PP), and post-docetaxel treatment. If patients die, they firstly go through the ‘BSC before death’ phase involving palliative care, until death. This consists of the ‘end-of-life’ phase where patients are near death and will not receive additional active treatments that may impact survival, but instead are managed for their pain or other symptoms. b BSC (PP) involves active monitoring without active treatments that impact survival (patients are still receiving treatments that palliate symptoms of disease, e.g. corticosteroids). c Patients for whom pre-docetaxel treatment was discontinued or in whom disease was progressed were monitored in a BSC (pre-docetaxel) phase prior to commencing docetaxel treatment. No active treatment that impacted survival was provided during this phase (although patients are still receiving treatments that palliate symptoms of disease). d Patients started docetaxel only if ECOG PS score <2 (assumed to correspond to Karnofsky PS score ≥60 %). Otherwise, patients moved to ‘BSC before death’ until death. e This phase involves no active treatment that has shown to impact overall survival while patients are still receiving treatments that palliate symptoms of disease. Furthermore, it was assumed that if patients received AAP prior to docetaxel they would not be eligible for AAP retreatment post-docetaxel, whereas BSC patients were allowed to receive AAP post-docetaxel