Abstract
Memory deterioration is the earliest and most devastating cognitive deficit in normal aging and Alzheimer’s disease. Some older adults, known as “Supernormals”, maintain excellent memory. This study examined relationships between cerebral amyloid deposition and functional connectivity (FC) within the cingulate cortex (CC) and between CC and other regions involved in memory maintenance between Supernormals, healthy controls, and those at risk for Alzheimer’s disease (amnestic mild cognitive impairment). Supernormals had significantly stronger FC between anterior CC and R-hippocampus, middle CC (MCC) and L-superior temporal gyrus, and posterior CC and R-precuneus, while weaker FC between MCC and R-middle frontal gyrus and MCC and R-thalamus than other groups. All of these FC were significantly related to memory and global cognition in all participants. Supernormals had less amyloid deposition than other groups. Relationships between global cognition and FC were stronger among amyloid positive participants. Relationships between memory and FC remained regardless of amyloid level. This revealed how CC-related neural function participates in cognitive maintenance in the presence of amyloid deposition, potentially explaining excellent cognitive function among Supernormals.
Keywords: Supernormal, memory, cingulate cortex, cerebral amyloid deposition, functional connectivity
Introduction
Deterioration of cognition, particularly episodic memory, is believed to be an inevitable part of the aging process that is accelerated in Alzheimer’s disease (AD) (Tromp, Dufour, Lithfous, Pebayle, & Despres, 2015). Recent literature suggests inter-individual variability in maintaining memory capacity in old age (Nyberg, Lovden, Riklund, Lindenberger, & Backman, 2012; Raz & Lindenberger, 2011). Indeed, some older adults demonstrate superior memory capacity compared to cognitively normal counterparts at younger or similar age, and retain excellent memory capacity over decades (T. Gefen et al., 2015; Habib, Nyberg, & Nilsson, 2007; Rogalski et al., 2013). These “Supernormal” individuals are a unique population for studies as understanding the physiology supporting superior memory may provide insights for preservation of cognitive function during aging as well as treatment of age-related cognitive diseases such as AD (Mapstone et al., under review). Cerebral amyloid deposition, a signature AD pathology, occurs decades earlier than the clinical onset of AD, precipitating memory deterioration (Jack et al., 2010). It is unclear, however, whether any brain regions’ function is particularly resistant to AD pathology and whether this resistance to pathology contributes to superior memory exhibited by Supernormals.
Most neuroimaging investigations of memory deterioration in normal aging and age-associated neurodegeneration have focused on medial temporal/hippocampal and prefrontal disruptions (Corkin, 2002; Park & Reuter-Lorenz, 2009). A recent study also confirmed the discrete hippocampal and prefrontal activations in supporting Supernormals’ excellent memory capacity (Pudas et al., 2013). Meanwhile, the cingulate cortex (CC), given its low frequency of AD-related neurofibrillary tangles and preserved cortical thickness, appears to play a unique role in supporting Supernormals’ cognitive performance (T. Gefen et al., 2015; Rogalski et al., 2013). Cumulative evidence suggests the cingulate cortex is a hub that links multiple brain regions (Gong et al., 2009; Hagmann et al., 2008). By integrating input from various sources, such as hippocampus and prefrontal cortex, CC actively participates in the regulation of multiple cognitive functions as part of Papez circuit (or medial limbic circuit) (Papez, 1937; Shackman et al., 2011), and is disrupted early in both normal aging and AD-associated neurodegeneration (Chang et al., 2015; Sheline et al., 2010). Such characteristics make CC an ideal region for understanding the influence of functional connectivity (FC) on excellent memory, by connecting to the hippocampal and prefrontal regions. Furthermore, compared to other brain regions, including medial temporal/hippocampal and prefrontal lobes, CC is affected by amyloid deposition early in both aging and AD (Camus et al., 2012; Chang et al., 2015; La Joie et al., 2012). Notably, in an emerging structural imaging study examining the default neural networks, cortical thickness was preserved in multiple cingulate, hippocampal, and frontal regions among Supernormals when compared to their younger counterparts (Sun et al., 2016). In resting-state functional MRI (rs-fMRI) studies of cognitively normal (but not necessarily cognitively excellent) older adults, greater FC between anterior (ACC) and posterior CC (PCC), between ACC and prefrontal cortex, or between PCC and hippocampus have been correlated to higher education, often used as a surrogate for intelligence, and overall better cognitive function (Arenaza-Urquijo et al., 2013; Shu et al., 2016; Wang et al., 2010). Similarly, the reduction of ACC and/or PCC’s FC with other regions (e.g., precuneus, hippocampus, middle temporal lobe) has been consistently revealed in individuals with amnestic mild cognitive impairment (MCI) compared to their cognitively normal controls (Bai et al., 2009; Binnewijzend et al., 2012; Dunn et al., 2014; Tam et al., 2015; Yan, Zhang, Chen, Wang, & Liu, 2013), and differentiates individuals with amnestic MCI from MCI due to deficits unrelated to AD (Dunn et al., 2014). However, the relationships between FC and cerebral amyloid deposition have been varied across studies of cognitively normal older adults or MCI (Chao et al., 2013; Hedden et al., 2009; Lim et al., 2014; Mormino et al., 2011; Sheline et al., 2010; Sperling et al., 2009; Zhou, Yu, & Duong, 2015).
In the present study we examined the relationship between cerebral amyloid deposition and FC within discrete regions of CC (ACC, middle CC (MCC), and PCC) and more importantly, between CC and other regions involved in memory maintenance between Supernormals, healthy controls (HC), and older adults diagnosed with amnestic mild cognitive impairment (MCI), who are at high risk for AD. We hypothesized that, compared to HC or MCI, more efficient FC of CC would support superior memory abilities among Supernormals, even in the presence of amyloid.
Materials and Methods
ADNI dataset
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
Participants
The imaging and cognitive data were obtained from the ADNIGO and ADNI2 datasets since participants included in the present study were required to have 3T rs-fMRI and florbetapir (18F-AV-45) PET imaging data. Participants had clinical assessment visits to assess their cognitive and functional capacities that were separated from the imaging visits. We used the cognitive data from the clinical assessment visits with one-year interval. We first identified a group of cognitively normal older adults with superior memory using the following criteria: (1) being cognitively normal; and (2) having standardized episodic memory (EM) composite scores >1.5 across all of the available clinical assessment visits with at least one follow-up assessment in ADNIGO and ADNI2. Of note, the average standardized EM composite score (“norm data”) is 1 among the entire ADNI sample (see description in the “Measures” section below). We choose to use the term “Supernormal” in our work because our definition is based on an individual’s memory performance relative to age matched peers. Other definitions of older adults with superior cognitive abilities (e.g., (T. Gefen et al., 2015; Rogalski et al., 2013) are based on cognitive ability relative to younger cohorts. Thus, older subjects who perform as well as or better than younger subjects are termed “Superagers” implying a superior relative abilty across time. Whereas our term “Supernormals” is based in a statistical framework for a poplation distribution of the same age. In our opinion, each definition has merits, but the definitional differences should not be lost when comparing results across different studies.
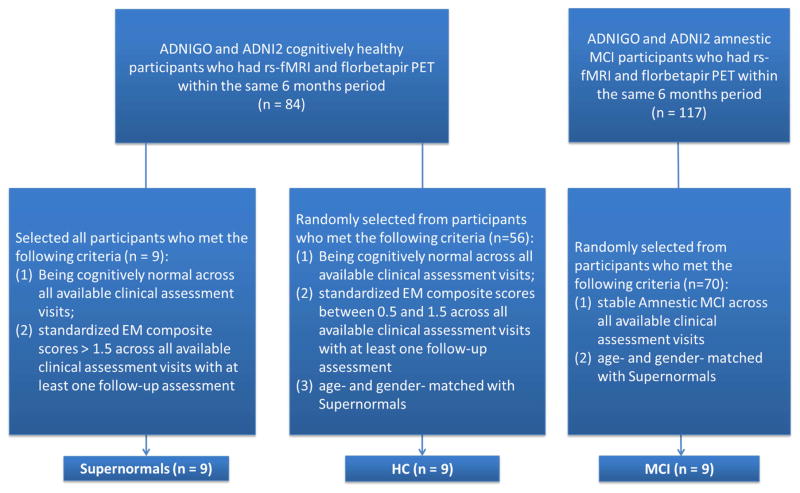
We also randomly identified two age- and gender-matched comparison groups (healthy control (HC), and amnestic MCI) from eligible participants in ADNIGO and ADNI2. The HC group was required to be cognitively normal and have standardized EM composite scores between 0.5 and 1.5 across all of the available clinical assessment visits and had at least one follow-up assessment in ADNIGO and ADNI2. The amnestic MCI group (“late MCI” as defined in ADNIGO and ADNI2) consisted of participants diagnosed by a psychiatrist or neurologist at study sites, and reviewed by a Central Review Committee, based on subjective memory complaint and impaired memory performance from serial neuropsychological tests (including the Logical Memory II subscale of the Wechsler Memory Scale-Revised, the Mini-Mental State Exam, and the Clinical Dementia Rating). Similar to the other two groups, the amnestic MCI group was required to remain in the same clinical category across all of the available clinical assessment visits and have at least one follow-up assessment in ADNIGO and ADNI2. More information about the diagnostic criteria for MCI and HC can be found in the literature (Petersen et al., 2010). Figure 1 displays the flowchart for the sample selection process.
Figure 1. Sample selection flow chart.
Note. Participants’ imaging visits were separated from their clinical assessment visits. EM = episodic memory; HC = healthy control; MCI = mild cognitive impairment.
Measures
Cognition
EM and executive function (EF) were measured using two composite scores developed based on serial factor analyses (Crane et al., 2012; Gibbons et al., 2012). The composite EM index was based on the memory-related domains of the Mini Mental Status Examination, Alzheimer’s Disease Assessment Scale-Cognition subscale, Rey Auditory Verbal Learning Test, and Logical Memory test. The composite EF index was based on the Wechsler Memory Scale- Revised Digit Span Test, Digit Span Backwards, Category Fluency, Trails A and B, and the Clock Drawing Test. Global cognition was measured using the Montreal Cognitive Assessment (MOCA) (Rossetti, Lacritz, Cullum, & Weiner, 2011). The baseline to year-4 follow-up longitudinal cognitive profile for the three groups is displayed in Supplemental Figure. In addition to the baseline, the average follow-up period ranged from 2.56 for Supernormal, 2.67 for HC, to 3.33 years for MCI without group differences (F = 1.72, p = .20). The three groups were significantly different in baseline and rate of change of EM (Wald’s χ2 = 91.92, p < .001; Wald’s χ2 = 11.42, p < .003, respectively) and MOCA (Wald’s χ2 = 6.10, p = .047; Wald’s χ2 = 15.07, p = .001, respectively), but not EF using the Generalized Estimating Equation model with the first-order autoregressive (AR(1)) working correlation matrix. This model takes into account the correlation between assessments at two consecutive time points and random errors, such as missing data, occurring at the individual level (Liang & Zeger, 1986). Of note, the cross-sectional cognitive data used in the present study were from the time points where imaging data for both modalities were available (48.1% were baseline visit with the rest being follow-up visits, see Table 1).
Table 1.
Group Comparison in the Background Characteristics
| Supernormal (n = 9) | HC (n = 9) | MCI (n = 9) | F or χ2 test (p value) | |
|---|---|---|---|---|
| Age, M (SD) | 73.53 (6.38) | 72.31 (5.57) | 72.97 (6.91) | 0.08 (.92) |
| Male, n (%) | 1 (11.1) | 1 (11.1) | 1 (11.1) | 0 (1.00) |
| Years of education, M (SD) | 17.11 (2.42) | 16.89 (2.03) | 14.78 (2.49) | 2.77 (.08) |
| EM, M (SD) | 1.83 (0.23) a | 1.03 (0.13) b | −0.08 (0.31) c | 118.68 (< .001) § |
| EF, M (SD) | 1.16 (0.85) | 0.73 (0.65) | 0.45 (0.79) | 0.85 (.44) § |
| MOCA, M (SD) | 28.22 (1.48) a | 25.56 (2.13) b | 21.44 (2.74) c | 16.54 (< .001) § |
| SUVR, M (SD) | 1.04 (0.09) a | 1.16 (0.17) a,b | 1.20 (0.12) b | 3.72 (.026) § |
| • SUVR+, n (%) | 2 (22.2) | 4 (44.4) | 7 (77.9) | 5.96 (.051) |
| • M (SD) for SUVR− group | 1.00 (0.05) | 1.04 (0.04) | 1.03 (0.01) | 1.11 (.36) |
| • M (SD) for SUVR+ group | 1.19 (0.04) | 1.32 (0.12) | 1.25 (0.09) | 1.45 (.28) |
Controlled for education.
means with different superscripts are significantly different from each other; means with the same superscript are not significantly different (Least Significant Difference's post hoc test, p <.05).
Imaging data acquisition, preprocessing, and analysis
FC
Participants included in the current study were scanned with a 3.0 Tesla Phillips MRI. The T1-weighted structural brain images were acquired using a magnetization-prepared rapid gradientwecho (MPRAGE) sequence (TE = 3.13 ms, TR = 6.77 ms, matrix = 256×256×170 in x-, y- and z-dimensions, voxel size = 1×1×1.2 mm3, flip angel = 9°). The rs-fMRI imaging data were obtained using an echo-planar imaging (EPI) sequence (TR = 3000 ms, TE = 30 ms, slice thickness = 3.3 mm, matrix = 64×64, voxel size = 3×3×3 mm3, number of volumes = 140, number of slices = 48). The rs-fMRI data were preprocessed using connectivity toolbox (Conn16a) (Whitfield-Gabrieli & Nieto-Castanon, 2012) based on statistical parametric mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/). For each participant, the first 10 volumes were removed to avoid potential noise related to the equilibrium of the scanner and participant’s adaptation to the scanner. The remaining 130 volumes were slice-timing and motion corrected. The images were then co-registered to each individual’s own anatomical scan, normalized to the Montreal Neurological Institute (MNI)152 space, and resampled at 2 mm3. At last, a Gaussian kernel (FWHM = 6mm) was applied to smooth all the images. Before FC analysis, the linear trend was removed and a band pass filter (0.01 – 0.08 Hz) was applied to reduce the physiological noise. Then the component-based analysis was applied to remove the cerebrospinal fluid, white matter, movement parameters, and time-series predictors of global signal (Behzadi, Restom, Liau, & Liu, 2007). Three regions of interest (ROIs), ACC, MCC, and PCC, were identified using automated anatomical labeling of 116 predefined anatomical brain regions. The ROI masks were generated for the FC analysis using resting-state fMRI data analysis toolkit (REST) (Song et al., 2011). For FC analysis, the averaged BOLD time course in each ROI was extracted as the seed and used to do voxel-wised whole brain connectivity, respectively. We applied one-way ANOVA to explore group differences in FC involved each ROI, with threshold at p<0.005, clusters>432 mm3, corrected p<0.01. In addition, we calculated the gray matter volumes of the three CC regions and for each ROI generated from the FC analysis, as well as the whole brain, using voxel-based morphometry analysis. We did not find any significant group difference in the gray matter volume after adjusting for multiple comparisons (all p > .05).
Florbetapir standardized uptake value ratio (SUVR)
Florbetapir PET images were downloaded from the ADNI database in their fully pre-processed form (AV45 Coreg, Avg, Standardized Image and Voxel Size), as well as each subject’s structural T1 MR images, as described above. T1 images were first segmented using Freesurfer (http://surfer.nmr.mgh.harvard.edu/) and registered to the MNI152 template at an isotropic resolution of 2 mm using the FMRIB Software Library (FSL v5.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). The results of Freesurfer segmentation were examined visually for topological defects, with manual editing performed to correct these defects. PET images were then co-registered to these T1 images in MNI152 space. SUVRs were computed for each ROI described above, as well as for all cortical regions, using the whole cerebellum as a reference. A “whole cortex” SUVR summary value was calculated by averaging all of the individual cortical SUVRs. A threshold value of SUVR>1.11 (SUVR+/−) was also set for the whole cortex summary value to separate amyloid positive (SUVR+) and negative (SUVR−) participants, based on prior recommendations (Landau et al., 2013). Of note, we measured cerebral SUVR instead of limiting it to CC given the whole-brain FC analysis (although taking CC as seeds) we conducted. Additionally, we calculated ACC, MCC, and PCC SUVR, and there was no group difference (all F < 1.12, p > .05).
Other data analysis
Data analyses were conducted using SPSS 22.0 (IBM Corporation, Armonk, NY). Group comparisons of sample characteristics were made using ANOVA for continuous variables or χ2 tests for categorical variables. Post-hoc analysis was conducted with Least Significant Difference analysis. Group comparison in FC and cognitive function by SUVR+/− levels were examined using independent t-test with the entire sample. Generalized Linear Models with an identity link and linear scale response were used to examine the interaction between FC and group on cognitive function (ycognitive function = β0 + β1Age + β2Education + β3Group + β4FC + β5Group × FC + ε), as well as the interaction between FC and SUVR+/− on cognitive function (ycognitive function = β0 + β1Age + β2Education + β3Group + β4FC + β5SUVR+/− + β6Group × SUVR+/− + ε). All tests with False Discovery Rate (FDR)-adjusted two-tailed p values less than 0.05 were considered significant. FDR-correction was applied to address multiple comparisons among FC.
Results
Group comparison of FC, SUVR, and cognitive function
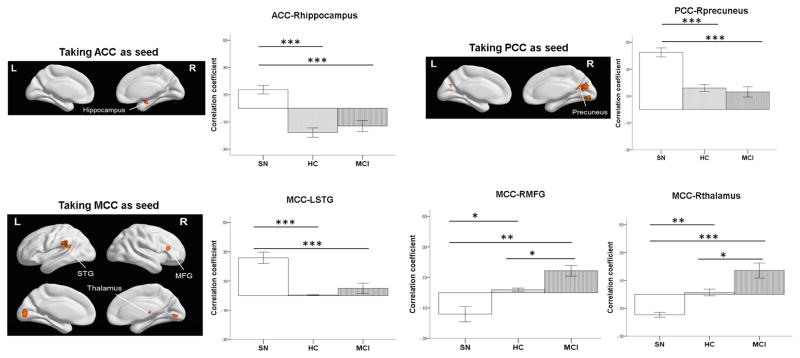
We placed seeds separately in the ACC, MCC, and PCC using voxel-wised whole brain connectivity with one-way ANOVA, and determined FC for five regions which distinguished Supernormal from the other groups. Supernormals had significantly stronger FC between ACC and right (R) hippocampus, MCC and left (L) superior temporal gyrus (STG), and PCC and R-precuneus (mean difference: 0.21 – 0.31, all p < .001), and weaker FC between MCC and R middle frontal gyrus (MFG) and MCC and R thalamus compared to the other groups (mean difference: −0.32 – −0.16, all p < .004); the MCI group had significantly stronger FC between MCC and R-MFG and MCC and R-thalamus than the other groups when controlling for education (mean difference: 0.12 – 0.32, all p < .022; see Figure 2). Of note, two FC of MCC or PCC with L-occipital cortex where HC were significantly stronger than the other two groups were excluded from the analysis. The three groups were significantly different in the EM and MOCA scores, and SUVR, but not in EF (see Table 1). The following analysis of cognitive function therefore only focused on EM and MOCA.
Figure 2. Group Differences in FC When Taking the Cingulate Cortex Regions as the Seeds.
SN = supernormal; HC = healthy control; MCI = mild cognitive impairment; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; MCC = middle cingulate cortex; STG = superior temporal gyrus; MFG = middle frontal gyrus. * p< .05; ** p< .01; *** p < .001. Education was controlled in the comparison.
Relationship between FC and cognitive function as total and by group
When controlling for age and education, stronger FC between ACC-Rhippocampus (r = .61, p = .001; r = .55, p = .005, respectively), MCC-LSTG (r = .60, p = .001; r = .56, p = .003, respectively), and PCC-Rprecuneus (r = .58, p = .002; r = .40, p = .046, respectively) and weaker FC in MCC-RMFG (r = −.78, p < .001; r = −.71, p < .001) and MCC-Rthalamus (r = −.75, p < .001; r = −.70, p < .001) were significantly related to better EM and MOCA in all participants. There was no correlation between FC and EF for the data taken as a whole. Additionally, the group did not affect the relationship between FC and cognitive function (i.e. no significant interaction effect between group and FC on cognitive function, see Supplemental Table 1).
Subsample analysis including only amyloid positive MCI
We excluded the two amyloid negative MCI cases (although both had consistent clinical amnestic MCI diagnosis over 3 or 5 years). The group differences in FC and cognitive domains remained the same as for the main analysis (see Supplemental Table 2). Group and RMFG (Wald’s χ2 = 9.97, p = .007) had significant interaction effects on memory. Group and MCC-Rthalamus (Wald’s χ2 = 10.49, p = .005) and PCC-Lprecuneus (Wald’s χ2 = 12.07, p = .002) had significant interaction effects on MOCA (see Supplemental Table 3).
Relationship between FC, SUVR, and cognitive function
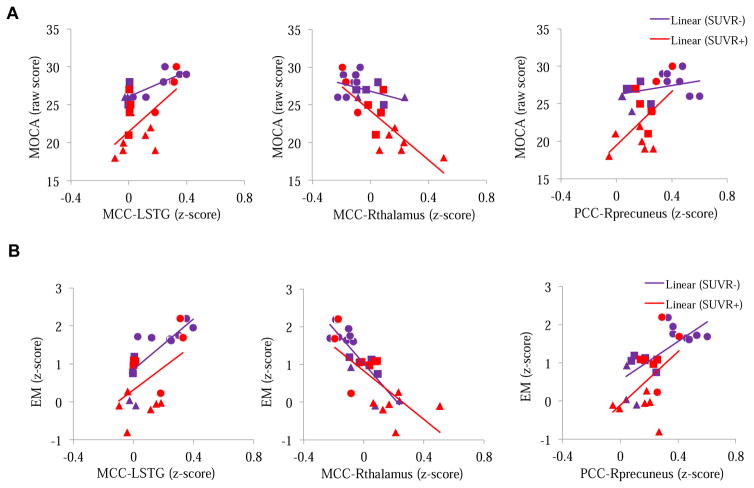
For the entire sample, the five FC did not differ based on SUVR+/− (all FDR-corrected p > .05); however, SUVR+ subjects had significantly lower levels of memory (t = 2.32, p = .044) and MOCA (t = 3.64, p = .006) than SUVR− subjects. Controlling for age, education, group, FC, and SUVR+/−, there were significant interaction effects between SUVR+/− and MCC-LSTG (Wald’s χ2 = 4.87, p = .027), MCC-Rthalamus (Wald’s χ2 = 12.94, p < .001), and PCC-Rprecuenues (Wald’s χ2 = 14.00, p < .001) on MOCA after FDR-correction. Compared to those who were SUVR−, individuals who were SUVR+ had a more positive relationship between MCC-L-STG and MOCA (B (SE) = 9.16 (4.15), Wald’s χ2 = 4.87, p = .027), and between PCC-Rprecuenues and MOCA (B (SE) = 14.02 (3.75), Wald’s χ2 = 14.00, p < .001), and more negative relationship between MCC-Rthalamus and MOCA (B (SE) = −13.66 (3.80), Wald’s χ2 = 12.94, p < .001) (see Figure 3a). There was no interaction effect on EM (Figure 3b is displayed as a comparison).
Figure 3. Relationship between FC and Cognitive Function (A. MOCA; B. EM) Separated by SUVR+/.
Different symbols represent different groups; triangle: MCI, square: HC, and circle: Supernormal. SUVR = standardized uptake value ratio; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; MCC = middle cingulate cortex; STG = superior temporal gyrus; MFG = middle frontal gyrus; EM = episodic memory; MOCA = Montreal Cognitive Assessment.
Discussion
We identified a group of older adults with excellent memory and global cognition and examined the role of CC involved FC in supporting the cognitive function, and its relationship with amyloid deposition. There were two lines of findings: First, when applying the regions of CC as seeds separately, the Supernormals had significantly stronger FC between ACC and R-hippocampus, MCC and L-STG, and PCC and R-precuneus, and weaker FC between MCC and R-MFG and MCC and R-thalamus than their age- and sex-matched cognitively normal or at-risk counterparts. Furthermore, stronger FC between ACC and R-hippocampus, MCC and L-STG, and PCC and R-precuneus, and weaker FC between MCC and R-MFG and MCC and R-thalamus were significantly related to better memory and global cognition in the entire sample. Second, the Supernormals had significantly lower levels of amyloid deposition. Across all groups, the relationships between global cognition and FC (including MCC and L-STG, MCC and R-thalamus, and PCC and R-precuneus) were stronger among those participants defined as amyloid positive. Conversely, the relationship between memory and FC remained regardless of the level of amyloid deposition.
Several FCs between CC and other brain regions that distinguish the Supernormals from their counterparts have been studied in the healthy aging or AD literature. Two of the regions showing stronger connectivity to CC in Supernormals (R-hippocampus and R-precuneus) are involved in episodic memory and the default mode network (DMN). As a number of studies have examined the relationship between aging and episodic memory (Tromp, et al., 2015), aging and DMN acitivity (Greicius, Srivastava, Reiss, & Menon, 2004), or brain volume within DMN among Supernormals (Sun et al., 2016), our results further validate the brain function and episodic memory in the Supernormals. For example, decreased FC between PCC and precuneus occurs in the early stages of AD (Binnewijzend et al., 2012; Zhang et al., 2009), and positive relationships between memory performance and FC in ACC and hippocampus were found in cognitively normal older adults (Arenaza-Urquijo et al., 2013; Li et al., 2014). On the other hand, the FC between MCC and two other regions (R-MFG and R-thalamus) is less studied, with the present study being the first to reveal FC of MCC with other cortical or subcortical regions linked to cognitive function. The MCC and thalamus are often studied independently in the stress regulation process (Kogler et al., 2015); MCC and MFG have also been studied separately in memory or learning tasks (Gould, Brown, Owen, Bullmore, & Howard, 2006), with an interesting case-study showing that the RMFG may play a role in the shifting of attention from exogenous to endogenous control (Japee, Holiday, Satyshur, Mukai, & Ungerleider, 2015). Noticeably, cumulative evidence suggests that cognitive protection against aging (as seen in HC group) or neurodegeneration (as seen in aMCI patients) is regulated through compensatory neural reconfigurations that rely heavily on recruitment of frontal regions due to the dysfunctional hippocampus (Bondi, Houston, Eyler, & Brown, 2005; Bookheimer et al., 2000; Park & Reuter-Lorenz, 2009). The distinct FC patterns observed in Supernormals when comparing to HC or MCI, such as stronger FC between ACC and R-hippocampus while weaker FC between MCC and R-MFG, may represent exceptional neural reserve or efficiency in Supernormals that violate the common neural deficit (e.g., hippocampus) or compensation (e.g., prefrontal cortex) seen in aging or neurodegeneration.
Supernormals seemed to have less cerebral amyloid deposition, and amyloid positive participants in general had significantly worse memory and global cognition. However, the lack of a significant relationship between amyloid deposition and FC involved the CC may be due to the relatively small sample. Of note, in the healthy aging literature, amyloid deposition has been shown to directly affect memory performance (Sperling et al., 2013), while functional changes in CC are highly correlated with amyloid retention (Hedden et al., 2009; Sheline et al., 2010; Sperling et al., 2009). Specifically, increased amyloid deposition has been shown to correlate with decreased FC in the DMN (Mormino, et al., 2011). However, the literature reports inconsistent results on amyloid deposition in Supernormals, with some investigators observing similar amyloid levels compared to controls (Tamar Gefen et al., 2014) and others reporting reductions in Supernormals (Imhof et al., 2007; Rogalski et al., 2013). Quantification of amyloid deposition in these studies was performed using samples from autopsied brains, rather than through PET imaging. The supernormal literature also shows inconsistent relationships between AD pathology and memory performance (Balasubramanian, Kawas, Peltz, Brookmeyer, & Corrada, 2012; Harrison, Weintraub, Mesulam, & Rogalski, 2012). The findings related to amyloid deposition with the group, FC, or cognition in the present study will need to be reexamined in large sample size. Also, there may be different mechanisms underlying the supernormal phenomenon, which require further clarification incorporating the examination of relevant structural connectivity.
The interactions between FC and amyloid deposition influenced memory and global cognition in slightly different ways. The stronger FC in Supernormals, which was related to their excellent cognitive function, was associated with memory; and the level of amyloid deposition did not affect this relationship. Conversely, FC, especially in MCC-LSTG, MCC-Rthalamus, and PCC-Rprecuneus, had stronger associations with global cognition in individuals with higher levels of amyloid deposition. This suggests that individuals with higher levels of amyloid deposition, who were less likely in the Supernormal group, may be more sensitive to dysfunctional FC, which results in a higher likelihood of deficits in global cognition. Noticeably, the significant interaction effects between FC and group on cognition only existed when restricting amnestic MCI group to those who were amyloid positive. Such findings were consistent with the interaction effects found between FC and amyloid deposition described above, which suggest CC-involved neural function may protect against AD pathology, instead of the clinical phenotypes. Neuroplasticity is inducible in CC such that the cingulate cortex can be modified by life style or behavioral factors (Lin et al., 2016), and the activation and functional connectivity of ACC and PCC involved network (e.g. DMN) are critical in maintaining cognitive reserve (Bozzali et al., 2015; Sumowski, Wylie, Deluca, & Chiaravalloti, 2010). This makes CC a viable target for intervention aimed to prevent memory decline or enhance memory capacity.
We acknowledge several limitations related to this small sample size preliminary work. First, although we also found insignificant group difference in EF in a different cohort study (Mapstone et al., under review), EF is known to be mediated by CC (Grambaite et al., 2011). The lack of significant results in group difference in EF or the correlation between EF and CC’s function may be due to the small sample size. Similar issues were also observed in the lack of significant group difference in amyloid deposition or brain volume in CC. The next step will be to solve these remaining questions with larger sample size. In addition, a fuller understanding of the group effect on relationships between amyloid deposition, cognitive function, and FC, as well as the correspondence of functional-structural connectivity in these connected regions, may help further clarify the impact of amyloid deposition on cognitive function. Next, the Supernormal phenomenon is an emerging concept in the cognitive aging field. We defined Supernormals completely based on individuals’ memory performance due to the vulnerability of memory in the AD-associated neurodegenerative process (Tromp et al., 2015). As described previously, Supernormality was defined based on a statistical distribution of cognitive performance in an aging population. It will be important to fully describe the Supernormal cognitive profile across multiple domains and determine the most appropriate cut-off scores by linking to relevant brain function and pathology data.
In conclusion, the present study revealed how CC-related neural function participates in the maintenance of cognitive function even in the presence of amyloid deposition. Such a process may help explain the excellent cognitive function among Supernormals.
Supplementary Material
Acknowledgments
The manuscript preparation was funded by the Alzheimer’s Association New Investigator Grant (NIRG-14-317353) and NIH R01 grant (NR015452) to F. Lin.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mezenge F, Perrotin A, … Chetelat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Bai F, Watson DR, Yu H, Shi Y, Yuan Y, Zhang Z. Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res. 2009;1302:167–174. doi: 10.1016/j.brainres.2009.09.028. S0006-8993(09)01930-1 [pii] [DOI] [PubMed] [Google Scholar]
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79(9):915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. S1053-8119(07)00383-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewijzend MA, Schoonheim MM, Sanz-Arigita E, Wink AM, van der Flier WM, Tolboom N, Barkhof F. Resting-state fMRI changes in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2012;33(9):2018–2028. doi: 10.1016/j.neurobiolaging.2011.07.003. S0197-4580(11)00270-3 [pii] [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.wnl.0000150885.00929.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/nejm200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Dowling C, Serra L, Spano B, Torso M, Marra C, Cercignani M. The impact of cognitive reserve on brain functional connectivity in Alzheimer's disease. J Alzheimers Dis. 2015;44(1):243–250. doi: 10.3233/JAD-141824. P4725RK22K8031M9 [pii] [DOI] [PubMed] [Google Scholar]
- Camus V, Payoux P, Barré L, Desgranges B, Voisin T, Tauber C, … Guilloteau D. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39:621–631. doi: 10.1007/s00259-011-2021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YT, Huang CW, Chang YH, Chen NC, Lin KJ, Yan TC, … Chang CC. Amyloid burden in the hippocampus and default mode network: relationships with gray matter volume and cognitive performance in mild stage Alzheimer disease. Medicine (Baltimore) 2015;94(16):e763. doi: 10.1097/md.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, DeCarli C, Kriger S, Truran D, Zhang Y, Laxamana J, … Mack WJ. Associations between white matter hyperintensities and β amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PloS one. 2013;8(6):e65175. doi: 10.1371/journal.pone.0065175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3(2):153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Mungas D. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6(4):502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CJ, Duffy SL, Hickie IB, Lagopoulos J, Lewis SJ, Naismith SL, Shine JM. Deficits in episodic memory retrieval reveal impaired default mode network connectivity in amnestic mild cognitive impairment. Neuroimage Clin. 2014;4:473–480. doi: 10.1016/j.nicl.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Papastefan S, Peterson M, Kim G, Rogalski E, Weintraub S, … Geula C. Histopathologic substrates of cingulate integrity in superaging: a stereological study. Neurobiol Aging. 2014;35:718. [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, … Geula C. Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci. 2015;35(4):1781–1791. doi: 10.1523/jneurosci.2998-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Crane PK. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19(3):524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Bullmore ET, Howard RJ. Task-induced deactivations during successful paired associates learning: an effect of age but not Alzheimer's disease. Neuroimage. 2006;31(2):818–831. doi: 10.1016/j.neuroimage.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Grambaite R, Selnes P, Reinvang I, Aarsland D, Hessen E, Gjerstad L, Fladby T. Executive dysfunction in mild cognitive impairment is associated with changes in frontal and cingulate white matter tracts. J Alzheimers Dis. 2011;27(2):453–462. doi: 10.3233/JAD-2011-110290. CM5777636862248H [pii] [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [doi] 0308627101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Nilsson LG. Cognitive and non-cognitive factors contributing to the longitudinal identification of successful older adults in the betula study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14(3):257–273. doi: 10.1080/13825580600582412. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, Rogalski E. Superior memory and higher cortical volumes in unusually successful cognitive aging. J Int Neuropsychol Soc. 2012;18(6):1081–1085. doi: 10.1017/s1355617712000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686–12694. doi: 10.1523/jneurosci.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Kövari E, Gunten Av, Gold G, Rivara CB, Herrmann FR, … Gianaakopoulos P. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007;257:72–79. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. S1474-4422(09)70299-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress - Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, … Chetelat G. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer's disease dementia. J Neurosci. 2012;32(46):16265–16273. doi: 10.1523/jneurosci.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, Mintun MA. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54(1):70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD, … Li SJ. Aberrant functional connectivity in Papez circuit correlates with memory performance in cognitively intact middle-aged APOE4 carriers. Cortex. 2014;57:167–176. doi: 10.1016/j.cortex.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Lim HK, Nebes R, Snitz B, Cohen A, Mathis C, Price J, … Aizenstein HJ. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137(Pt 12):3327–3338. doi: 10.1093/brain/awu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Heffner KL, Ren P, Tivarus ME, Brasch J, Chen DG, … Tadin D. Cognitive and Neural Effects of Vision-Based Speed-of-Processing Training in Older Adults with Amnestic Mild Cognitive Impairment: A Pilot Study. J Am Geriatr Soc. 2016;64(6):1293–1298. doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Lin F, Nalls M, Cheema A, Singleton A, Fiandaca MS, Federoff HJ. Plasma Metabolomics Reveal Evidence of Neuroplasticity in Successful Cognitive Aging and its Failure. Alzheimer’s Disease under review. [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Jagust WJ. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21(10):2399–2407. doi: 10.1093/cercor/bhr025. bhr025 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of neurology and psychiatry. 1937;38(4):725. [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Weiner MW. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudas S, Persson J, Josefsson M, de Luna X, Nilsson LG, Nyberg L. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci. 2013;33(20):8668–8677. doi: 10.1523/jneurosci.2900-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: cross-sectional studies offer no solution to the age-brain-cognition triangle: comment on Salthouse (2011) Psychol Bull. 2011;137(5):790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, … Mesulam MM. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci. 2013;25(1):29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77(13):1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, Shi Y, Chen G, Wang Z, Liu D, Yue C, … Zhang Z. Opposite Neural Trajectories of Apolipoprotein E 4 and 2 Alleles with Aging Associated with Different Risks of Alzheimer's Disease. Cereb Cortex. 2016;26(4):1421–1429. doi: 10.1093/cercor/bhu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, … Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, … Pontecorvo MJ. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013;34(3):822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, … Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(Pt 2):362–374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC. Youthful Brains in Older Adults: Preserved Neuroanatomy in the Default Mode and Salience Networks Contributes to Youthful Memory in Superaging. J Neurosci. 2016;36(37):9659–9668. doi: 10.1523/jneurosci.1492-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A, Dansereau C, Badhwar A, Orban P, Belleville S, Chertkow H, … Bellec P. Common Effects of Amnestic Mild Cognitive Impairment on Resting-State Connectivity Across Four Independent Studies. Front Aging Neurosci. 2015;7:242. doi: 10.3389/fnagi.2015.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp D, Dufour A, Lithfous S, Pebayle T, Despres O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res Rev. 2015;24(Pt B):232–262. doi: 10.1016/j.arr.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O'Keefe K, Putcha D, Bakkour A, Van Dijk KR, … Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51(2):910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yan H, Zhang Y, Chen H, Wang Y, Liu Y. Altered effective connectivity of the default mode network in resting-state amnestic type mild cognitive impairment. J Int Neuropsychol Soc. 2013;19(4):400–409. doi: 10.1017/S1355617712001580. S1355617712001580 [pii] [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res. 2009;197(1):103–108. doi: 10.1016/j.bbr.2008.08.012. S0166-4328(08)00435-X [pii] [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yu F, Duong TQ. White matter lesion load is associated with resting state functional MRI activity and amyloid PET but not FDG in mild cognitive impairment and early Alzheimer's disease patients. J Magn Reson Imaging. 2015;41(1):102–109. doi: 10.1002/jmri.24550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.