Abstract
CONTEXT
STDs are common among older adolescents and young adults; hence, STD screening is a public health priority. Home-based STD testing could be a strategy to improve screening rates, particularly among at-risk populations, including sexual minority (i.e., nonheterosexual) young adults.
METHODS
Data were collected from a national sample of 971 sexual minority young adults aged 18–26 through an online survey in the fall of 2013. Logistic regression analyses identified associations between respondents’ characteristics and their willingness to use a home-based test for chlamydia and gonorrhea.
RESULTS
A greater proportion of men than of women were willing to use a home-based STD test (81% vs. 68%). Willingness was more likely among gay than among bisexual men, among men insured through their parents than among the uninsured and among those who had had two or more sexual partners in the past year than among those who had had fewer (adjusted odds ratios, 2.0–2.2). Among men, students were less likely than the employed to report willingness for home-based testing (0.4). Among women, willingness was more likely among those who reported at least two partners in the past year than among those who reported fewer (1.6). Overall, respondents’ most common concerns about home-based STD testing regarded test accuracy, their ability to do the test correctly and their preference to see a doctor for testing.
CONCLUSIONS
Home-based STD testing may be a promising strategy for screening sexual minority young adults; understanding correlates of willingness and young adults’ concerns may help inform self-testing programs.
Bacterial STDs, including chlamydia and gonorrhea, represent a significant public health problem, as almost two million cases are reported each year in the United States.1 This figure likely underestimates the true burden, because many infections are asymptomatic and may go undiagnosed.2,3 STDs are implicated in a range of serious health outcomes, including pelvic inflammatory disease and infertility,4 as well as an increased risk of HIV infection.5 They impose substantial costs on the health care system6 and are associated with psychosocial difficulties such as stigma, shame and anxiety.7,8 Adolescents and young adults experience a greater risk of STDs than do other age-groups, as nearly half of all new infections occur among 15–24-year-olds.9,10 Consistent with trends from previous years, among women in 2012, those aged 15–19 and 20–24 had the highest rates of both chlamydia (3,291 and 3,695 cases per 100,000 females, respectively) and gonorrhea (512 and 579 cases per 100,000).1 Rates are lower among men than among women; among males, chlamydia and gonorrhea rates were highest for 20–24-year-olds (1,350 and 463 cases per 100,000, respectively).1
Sexual minority (i.e., nonheterosexual) populations experience a number of health disparities related to STDs and sexual health. Overall, sexual minority young adults engage in more sexual risk behaviors than do their heterosexual peers.11,12 Although current reportable disease surveillance systems do not routinely collect data on sexual orientation, previous research suggests that bisexual young adults of both genders are more likely than their heterosexual counterparts to report any STD diagnosis.12–14 Gay and bisexual men, in particular, are at increased risk of having an STD, including chlamydia and gonorrhea; furthermore, HIV-positive men are more likely than others to have these STDs.1 While gay and bisexual males’ elevated STD risk is widely acknowledged, sexual minority women are an often overlooked population, who may incorrectly perceive their risk as low.14,15 Not only can some STDs be transmitted between female sex partners, but many women with such partners also have had or still have male partners, which increases their risk of exposure.16,17 Existing data suggest that sexual minority women are vulnerable to chlamydia and gonorrhea infection;12 indeed, young adult bisexual women have been found to have a higher prevalence than their heterosexual counterparts.14
Because of the high prevalence of bacterial STDs and the potentially asymptomatic nature of infections, routine screening is critical. The Centers for Disease Control and Prevention recommends annual chlamydia and gonorrhea screening for all sexually active women aged 25 or younger.18 Its guidelines also recommend routine screening for common bacterial STDs among sexually active men who have sex with men; guidelines include at least annual testing for urethral, pharyngeal and rectal infection with chlamydia or gonorrhea (as indicated on the basis of reported sexual behaviors), and more frequent screening for men at increased risk.18 In emphasizing the public health importance of STD screening, Healthy People 2020 includes objectives to lower rates of chlamydia and gonorrhea among both males and females, and to increase chlamydia screening at the population level among sexually active females aged 20–24 to 80%;19 it does not, however, include a screening objective specifically for males.
Yet despite guidelines, current data suggest that many young adults are not receiving the recommended testing. For example, estimates of STD testing among females range from 18% among sexually active 12–19-year-olds in a national, population-based sample in 199520 to 52% among sexually active women aged 16–20 and 60% among those aged 21–24 who were enrolled in Medicaid in 2008.19 A 2002 study found that just 16% of asymptomatic males aged 18–19 and 17% of those aged 22–26 reported STD testing in the past year; among symptomatic males, 16% and 24%, respectively, reported such testing.21 Data on use of health services by sexual orientation are rare, but research suggests that screening rates among sexual minority youth are likely to be even lower than that among women overall. For example, lesbian and bisexual women are less likely than heterosexual women to report use of reproductive health services.12,13,22 Furthermore, research suggests that health care providers may not discuss sexual orientation and behavior with patients, thus preventing the accurate assessment of risk and provision of appropriate care;23,24 specifically, they may underestimate STD risk among sexual minority women.25
Low screening rates point to the need for novel approaches to increase STD testing, and self-testing may be an acceptable and effective strategy. Previous research suggests that low levels of testing may be due to a number of reasons, including low levels of knowledge about STDs and available services; misperceptions of risk; wait times for appointments; cost; shame or unwillingness to disclose risky sexual behavior to a health care provider; discrimination; and fear regarding specimen collection.26,27 Self-testing may help address many of these barriers and concerns, as it typically involves a patient’s collecting a specimen, through either a swab (vaginal, urethral or rectal) or a sample of first-void urine. The self-collection of samples has been found to be a valid and reliable method of detecting chlamydia and gonorrhea, and it compares well with collection by clinicians.28–30 For example, among women, chlamydia testing using self-collected vaginal swabs has been found to be more sensitive than that using clinician-collected endocervical swabs (97% vs. 88%) and to have equal specificity (99.9% vs. 100%).30 Among men who have sex with men, reports of sensitivity and specificity using self-collected rectal swabs have also been high (for chlamydia, 88% and 98%, respectively; for gonorrhea, 88% and 99%), as well as consistent with clinician-collected samples (98% for both).29 Previous research examining the acceptability of self-collected samples in clinic settings has shown that men and women, even adolescents, find the tests acceptable and easy to perform; moreover, many young women prefer this approach over pelvic examinations.31–35
Self-collecting samples opens up opportunities for STD testing outside of traditional settings. Self-collection that can be performed at home (i.e., home-based testing) allows individuals to collect specimens privately, at a time and place that is convenient for them, and then mail the specimen to a doctor or lab for testing. Home-based testing could be a particularly useful strategy for reaching populations that may need frequent screening or do not regularly access health services. Indeed, research has shown that home-based STD testing is acceptable and feasible, and improves screening rates.33,36–38 Yet, to our knowledge, few studies have examined the acceptability of home-based testing among young adults, and we know of none that focus on sexual minority women. An examination of home-based testing for sexual minority populations may be particularly important because of the elevated STD risk and reduced health care use among these groups.
In the present study, we examine lesbian, gay and bisexual young adults’ experience with STD testing and their willingness to use home-based tests. We also explore their concerns about home-based testing. Our aim is to provide data that can inform future programs designed to increase appropriate STD testing among these populations.
METHODS
Study Design
We used data from an online survey conducted in October and November 2013 with a national sample of young adults.39,40 Respondents were recruited from a voluntary research panel constructed by Harris Interactive through a variety of recruitment strategies, both online (e.g., targeted e-mails, banner placement on social media) and offline (e.g., postal mail invitations, telephone). The panel, which includes respondents throughout the United States and reflects the U.S. population on several demographic characteristics, contains a subset of lesbian, gay, bisexual and transgender individuals.41,42 In exchange for completing surveys, respondents receive points that can be redeemed for rewards. Individuals were eligible to participate in our survey if they were aged 18–26; lived in the United States; and identified themselves as lesbian, gay, bisexual or trans-gender in response to a screening question. The survey took an average of 17 minutes to complete. The institutional review board at The Ohio State University approved the study.
Of 2,014 individuals who were eligible to participate, 1,005 provided informed consent and completed the survey. We examined data from 971 male and female respondents who defined themselves as gay, lesbian or bisexual; we excluded data from 34 transgender individuals because of concerns about small cell sizes. Respondents in the current study were from 49 states and the District of Columbia.
Measures
STD home-based testing
Prior to questions about home-based testing, the survey provided a brief description of a hypothetical test that would detect gonorrhea and chlamydia infection; that people could do by themselves at home; and that would involve collecting a urine specimen or using a swab to get a genital specimen that is then mailed to a doctor for testing. Survey items then assessed whether respondents had ever used a home test for STDs and respondents’ willingness to use such a test in the future (“definitely not willing,” “probably not willing,” “not sure,” “probably willing” and “definitely willing”). We classified respondents as willing (if they said definitely or probably willing) or not willing (if they gave any other response). The survey then asked what concerns they would have about using a home-based test for STDs. Respondents could select multiple responses from a prepopulated list of potential concerns, including an open-ended response for “other” concerns.
Correlates
The survey assessed a number of demographic, health care, and sexual behavior and health characteristics. Demographic characteristics were age (18–21 or 22–26), race or ethnicity (white, black, Hispanic, other), education level (less than college, some college, at least a college degree), relationship status (unmarried vs. married or cohabiting),* employment status (employed, not employed, in school), annual household income (less than $50,000 or at least that amount), residence (rural, suburban, urban) and geographic region (East, Midwest, South, West).
Health care questions assessed respondents’ health insurance status (has no insurance, is insured through work or school, is insured through parent’s plan), receipt of a routine checkup in the past year, disclosure of sexual orientation to their health care provider and perception of ever having been discriminated against by a provider because of their sexual orientation. Sexual behavior and health questions assessed age at sexual debut (17 or younger vs. 18 or older), number of sexual partners in the past year (0–1 vs. two or more), whether respondents had ever had any test for STDs (“such as gonorrhea, chlamydia or syphilis, not including HIV”), whether they had ever been told by a provider that they had an STD and whether they had ever tested positive for HIV.
Analysis
We used chi-square tests to examine differences in respondent characteristics by gender and sexual identity. Then, using logistic regression analysis, we identified correlates of respondents’ willingness to use a home-based STD test. Given the suggested gender differences in STD risk and use of services,21,24 we assessed this among men and women separately. All variables associated at p<.10 in bivariate regression analyses were included in a multivariate model to produce adjusted odds ratios and 95% confidence intervals. Finally, we used chi-square tests to determine if concerns about home-based testing differed by gender and sexual identity. We analyzed data in Stata/IC version 13. Statistical tests were two-tailed with a critical alpha of 0.05.
RESULTS
Sample Characteristics
Overall, slightly more than half of respondents were women (56%) and most were 22–26 years old (68%—Table 1). Smaller proportions of men than of women self-identified as bisexual (28% vs. 74%), were married or living with a partner (17% vs. 35%) and were unemployed (12% vs. 20%). Larger proportions of men than of women were Hispanic (18% vs. 14%) and reported an annual household income of at least $50,000 (35% vs. 26%).
TABLE 1.
Percentage distribution of a sample of gay, lesbian and bisexual young adults, by selected characteristics, according to gender, United States, 2013
| Characteristic | Overall | Men | Women |
|---|---|---|---|
| ALL | (N=971) | (N=428) | (N=543) |
| Sexual identity*** | |||
| Gay/lesbian | 46 | 72 | 26 |
| Bisexual | 54 | 28 | 74 |
| Age | |||
| 18–21 | 32 | 29 | 33 |
| 22–26 | 68 | 71 | 67 |
| Race/ethnicity* | |||
| White | 66 | 64 | 68 |
| Black | 9 | 7 | 10 |
| Hispanic | 16 | 18 | 14 |
| Other | 10 | 11 | 8 |
| Education | |||
| <college | 20 | 17 | 21 |
| Some college | 37 | 36 | 38 |
| ≥college | 44 | 47 | 41 |
| Relationship status*** | |||
| Unmarried | 73 | 83 | 65 |
| Married/cohabiting | 27 | 17 | 35 |
| Employment status*** | |||
| Employed | 52 | 55 | 50 |
| Not employed | 17 | 12 | 20 |
| In school | 31 | 33 | 31 |
| Annual household income*** | |||
| <$50,000 | 60 | 54 | 65 |
| ≥$50,000 | 30 | 35 | 26 |
| Not reported | 9 | 11 | 8 |
| Residence | |||
| Rural | 18 | 16 | 20 |
| Suburban | 43 | 46 | 41 |
| Urban | 39 | 39 | 38 |
| Region | |||
| East | 24 | 21 | 27 |
| Midwest | 23 | 24 | 23 |
| South | 28 | 29 | 27 |
| West | 26 | 27 | 23 |
| Health insurance status | |||
| Not insured | 20 | 20 | 20 |
| Insured through work/school | 37 | 38 | 36 |
| Insured through parent’s plan | 43 | 42 | 43 |
| Had a routine checkup in past year* | |||
| No | 53 | 57 | 50 |
| Yes | 47 | 43 | 50 |
| Disclosed sexual orientation to provider | |||
| No | 70 | 68 | 72 |
| Yes | 30 | 32 | 29 |
| Ever discriminated against by provider | |||
| No | 82 | 81 | 83 |
| Yes | 8 | 8 | 8 |
| Don’t know | 10 | 11 | 9 |
| SEXUALLY EXPERIENCED | (N=844) | (N=367) | (N=477) |
| Age at sexual debut***,† | |||
| ≤17 | 58 | 48 | 67 |
| ≥18 | 42 | 52 | 33 |
| No. of sexual partners in past year***,‡ | |||
| 0–1 | 56 | 45 | 64 |
| ≥2 | 44 | 55 | 36 |
| Ever had an STD test** | |||
| No | 38 | 45 | 32 |
| Yes | 62 | 55 | 68 |
| Ever been told had an STD**,§ | |||
| No | 81 | 84 | 79 |
| Yes | 19 | 16 | 21 |
| Ever used a home test*** | |||
| No | 98 | 96 | 99 |
| Yes | 2 | 4 | 1 |
| Ever tested positive for HIV | |||
| No | 96 | 95 | 97 |
| Yes | 4 | 5 | 3 |
| Total | 100 | 100 | 100 |
p<.05.
p<.01.
p<.001.
At first vaginal, anal or oral sex.
Including vaginal, anal or oral sex.
Told by a health care provider.
Notes: Differences by gender were assessed in chi-square tests. Percentages may not total 100 because of rounding.
Most respondents were insured, through either work or school (37%) or a parent’s plan (43%). Men were less likely than women to have had a routine checkup in the past year (43% vs. 50%). Most respondents (82%) did not feel that they had been discriminated against by a provider because of their sexual orientation.
Overall, 87% of respondents reported ever having had sex. Among these individuals, greater proportions of men than of women were 18 or older at sexual debut (52% vs. 33%) and had had two or more sexual partners in the past year (55% vs. 36%). Sixty-two percent reported ever having had a test for STDs; men were less likely than women to have had one (55% vs. 68%). Notably, gay men were more likely than bisexual men to have had an STD test (58% vs. 46%—not shown), while lesbian women were less likely than bisexual women to have done so (56% vs. 73%). Nineteen percent of sexually experienced respondents said they had ever been told by a health care provider that they had an STD. The proportion differed significantly between men and women (16% vs. 21%), and also varied by sexual identity (not shown): Gay men were more likely than bisexual men to have received an STD diagnosis (19% vs. 7%), and lesbian women were less likely than bisexual women to have received one (6% vs. 26%). Only 2% of all sexually experienced respondents reported ever having used a home-based STD test; this proportion varied between men and women (4% vs. 1%).
Willingness to Use
Among all respondents, most (74%) were willing to use a home-based STD test, though a greater proportion of men than of women were willing to do so (81% vs. 68%; p<.001—Table 2). The vast majority of respondents (95%) who had previously used a home-based test said they would use one again (93% of men and 100% of women—not shown). Because of small cell sizes and lack of variation in responses, this measure was not included in logistic regression analysis.
TABLE 2.
Percentage of respondents willing to use a home-based test for STDs, by selected characteristics; and odds ratios (and 95% confidence intervals) from multivariate logistic regression analyses assessing associations between characteristics and willingness—all according to gender
| Characteristic | Men | Women | ||
|---|---|---|---|---|
|
| ||||
| % | Odds ratio | % | Odds ratio | |
| All | 81 | na | 68 | na |
| Sexual identity | ||||
| Gay/lesbian | 85 | 1.98 (1.16–3.40)* | 70 | na |
| Bisexual (ref) | 71 | 1.00 | 67 | na |
| Age | ||||
| 18–21 (ref) | 74 | 1.00 | 69 | na |
| 22–26 | 84 | 1.41 (0.76–2.63) | 67 | na |
| Education | ||||
| <college (ref) | 71 | 1.00 | 69 | na |
| Some college | 79 | 1.29 (0.63–2.61) | 68 | na |
| ≥college | 87 | 1.33 (0.61–2.91) | 67 | na |
| Employment status | ||||
| Employed (ref) | 87 | 1.00 | 66 | 1.00 |
| Not employed | 71 | 0.58 (0.26–1.29) | 63 | 0.91 (0.56–1.46) |
| In school | 76 | 0.44 (0.24–0.85)* | 73 | 1.47 (0.95–2.27) |
| Region | ||||
| East (ref) | 83 | na | 66 | 1.00 |
| Midwest | 78 | na | 59 | 0.72 (0.44–1.19) |
| South | 80 | na | 75 | 1.47 (0.88–2.45) |
| West | 83 | na | 70 | 1.16 (0.69–1.95) |
| Health insurance status | ||||
| Not insured (ref) | 71 | 1.00 | 75 | na |
| Insured through work/school | 84 | 1.32 (0.65–2.68) | 70 | na |
| Insured through parent’s plan | 83 | 2.22 (1.09–4.49)* | 65 | na |
| No. of sexual partners in past year† | ||||
| 0–1 (ref) | 74 | 1.00 | 64 | 1.00 |
| ≥2 | 89 | 2.04 (1.17–3.57)* | 79 | 1.59 (1.05–2.40)* |
p<.05.
Among sexually experienced respondents.
Notes: All characteristics shown in Table 1 were included in bivariate regression analyses; for each gender, only those variables with an association at p<.10 were included in a multivariate model and are shown here. ref=reference group. na=not applicable, because variable was not included.
Men
The following variables were included in the multivariate analyses: sexual identity, age, education, employment status, health insurance status and number of partners in the past year. Gay men were more likely than bisexual men to be willing to use a home-based STD test (odds ratio, 2.0), and men who were insured through a parent’s health plan were more likely than those without insurance to do so (2.2). Men who had had two or more sexual partners in the past year also had elevated odds of being willing (2.0). Finally, students were less likely than those who were employed to say they would use such a test (0.4).
Women
Employment status, region of residence and number of partners in the past year were included in the multivariate analyses. Only one measure was significant: Women who had had two or more partners in the past year were more likely than those who had had one or no partners to report a willingness to use the home-based STD test (odds ratio, 1.6).
Concerns
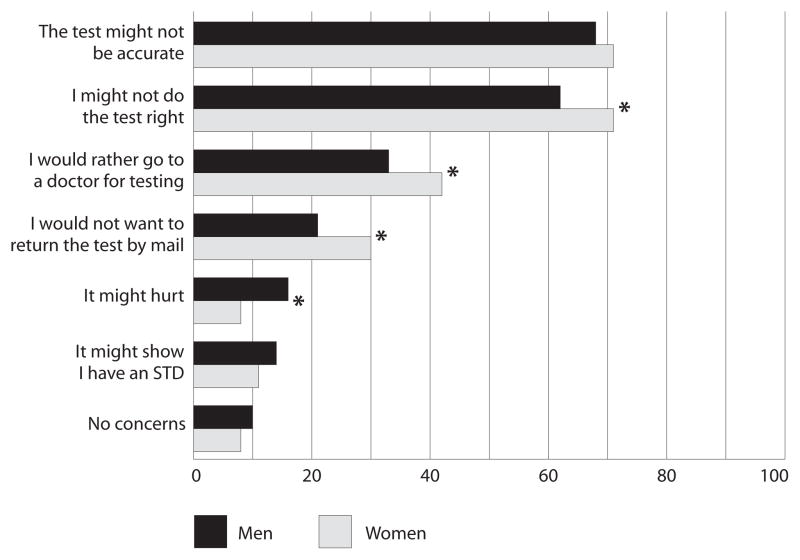
Overall, the most commonly reported concerns about home-based STD testing were the same for men and women; most respondents indicated that they would be concerned that the test might not be accurate (68% and 71%, respectively—Figure 1). However, significantly smaller proportions of men than of women felt that they might not do the test correctly (62% vs. 71%), would rather go to the doctor for an STD test (33% vs. 42%) or would not want to return the test by mail (21% vs. 30%); a larger proportion of men than of women were concerned that the test might hurt (16% vs. 8%). Small percentages of men and women were concerned that the test might show they had an STD (14% and 11%, respectively) or had no concerns about home-based testing (10% and 8%).
FIGURE 1. Percentages of respondents reporting specific concerns about using a home-based STD test, by gender.
*p<.05.
Additional analyses stratified by gender revealed a few significant differences in concerns about home-based STD testing by sexual identity (not shown). Gay men were more likely than bisexual men to be concerned that the test might not be accurate (73% vs. 54%) and to say they would rather go to a doctor for testing (37% vs. 24%). Finally, lesbian women were less likely than bisexual women to be concerned that they might not do the test correctly (64% vs. 73%).
DISCUSSION
Sexual minority populations are vulnerable to STDs;1,12–14 hence, STD screening and retesting among those who have been infected are critical. The current study provides important insight into the acceptability of and concerns about home-based STD testing among a national sample of sexual minority young adults. Our finding that most respondents (81% of men and 68% of women) were willing to use a home-based test is similar to previous findings on STD self-testing acceptability among adults.29,38,43 Furthermore, given that only 62% of sexually experienced respondents had ever had an STD test, it is encouraging that most, regardless of gender, were willing to use a test at home. Our findings suggest that home-based testing may be a viable strategy for reaching and screening sexual minority adults, including those without a history of STD testing. Importantly, home-based testing programs can be implemented through several avenues, such as distribution at community venues or online access,44 and this flexibility may increase access for populations that may not otherwise use sexual health services.
To our knowledge, this is the first study to examine correlates of home-based testing acceptability among sexual minority young adults. We found that willingness to use such a test was more common among respondents who had recently had multiple sexual partners than among others; indeed, this was the only consistent correlate across genders. Because the objective risk of STD infection increases with the number of sexual partners, this finding suggests that home-based testing may be most acceptable to young adults who are at greatest risk. Our measure of willingness to use a home-based test may also reflect respondents’ perceived risk of STDs (which is closely associated with their number of sexual partners45) and, thus, their perceived need for testing. Although additional bivariate associations were attenuated in the multivariate model for women, willingness to use a home-based STD test also varied by a number of demographic characteristics among men. For example, men who self-identified as gay were more willing than bisexual men to use a home-based test. This may reflect that previous STD testing was also more common among gay men—findings that are consistent with other research showing higher levels of HIV testing among gay than among bisexual men.46 Additional research is needed to understand the mechanisms underlying these associations (e.g., perceived risk), which could identify modifiable targets for STD testing programs.
Insurance coverage and perceived affordability of home-based STD testing may be particularly important issues to consider for young adults, given that they have lower levels of insurance coverage than other age-groups.47 While prices vary, end-user cost estimates of home-based STD tests available online range from free (for chlamydia and gonorrhea tests, available only to residents of specific states) to around $400 (for a full STD panel),48 excluding any follow-up and treatment for positive tests. In our study, men who were insured through a parent’s plan were more willing than those without insurance to use a home-based test. While it is logical that insurance coverage may make STD testing more affordable, the association with being covered on a parent’s plan, but not one’s own, is somewhat unexpected. Given that many young adults who have insurance through work or school are underinsured (e.g., have only minimal essential coverage and high out-of-pocket costs),47 this association could be due to the perception that their parents may have more comprehensive insurance that would cover STD testing. However, widely used billing procedures—such as sending detailed explanations of services to policyholders—could result in breaches of confidentiality for young adults accessing sexual health services through a parent’s insurance,49,50 and our study did not assess the awareness of potential implications, which could affect this association.
Participants also indicated their concerns with home-based STD testing, which provided valuable information that can help guide future programs promoting this screening method. Consistent with past studies,37,51 some of the most common concerns about home-based testing were about potential incorrect use, test accuracy and the need to return the test by mail. While our data do not identify which aspects of returning the test via mail concerned respondents, other research has found that confidentiality and potential delays in getting a diagnosis and treatment are concerns37,52 that could be related to mailing tests. Many of these reported concerns can be addressed by programs to promote home-based STD testing. For example, materials sent with tests should include clear instructions and indicate that most people who have used self-tests report that they are easy to use.32,38,53 Furthermore, targeting informational materials by gender and sexual orientation (e.g., through images, instructions and population-specific information about STD risks and potential concerns about home testing) may increase their relevance.54 Such materials offer a low-cost and sustainable strategy that may help promote home-based testing among sexual minority populations.
In addition to developing materials to promote home-based STD testing, it will be important to effectively communicate test results and ensure follow-up care and treatment. Few U.S. studies report information about these issues,44,55 though one study of an online, home-based testing program found that 40% of participants who returned specimens for testing took the initiative to call for results.35 In that study, staff needed to contact the remaining individuals to communicate test results, a step that can be labor-intensive and require heightened attention to confidentiality.55 However, research suggests that the vast majority of individuals who test positive do follow up and receive their results; the median follow-up across 11 studies was 96% (range, 67–100%).44 If home-based STD testing for sexual minority populations is to be effectively implemented, it will be important to identify effective and acceptable ways of ensuring notification of results and linkage with services for treatment.
Strengths and Limitations
Study strengths include a national sample of sexual minority young adults, populations that are at risk for disparities in sexual health outcomes and health care. Our study also had a large enough sample to allow us to examine potential differences by gender and sexual orientation.
Limitations include a sampling frame based on sexual identity rather than behavior, which may not be representative of all young adults who engage in same-sex sexual behavior. Our measures of sexual behavior and previous STD testing were based on self-report, though previous research supports the reliability of adolescents’ and young adults’ reports of sexual behavior and use of health services.56,57 Anonymous, Web-based administration may further increase the accuracy and reliability of the sensitive self-reported behavioral data collected in our study.58
Our measure of willingness to use a home-based STD test may overstate the actual use of self-testing if it is offered in practice, as intentions do not always translate into health behavior.59 In addition, survey items focused on home-based testing for chlamydia and gonorrhea, and did not examine acceptability of home-based testing for other STDs. The survey also did not assess respondents’ knowledge of home-based testing or cost, both of which could affect acceptability, or their preferences for specific types of tests (e.g., urine vs. swab). These are important areas for future research that can inform STD testing programs seeking to incorporate home-based testing. Additional research is also needed to examine willingness among specific sub-populations of sexual minority young adults, such as black men who have sex with men, who are disproportionately affected by chlamydia, gonorrhea and other STDs.1
This study also was limited by the lack of a heterosexual comparison group, the cross-sectional design and the modest response rate. Although we lack data on individuals who did not provide consent for our study, the online survey panel from which respondents were sampled is similar in composition to the U.S. population on several demographic characteristics.42
Conclusions
Wider use of home-based testing could improve STD screening rates and offers an alternative strategy that can augment traditional clinic-based testing. Findings from this national sample suggest that home-based testing could be an acceptable strategy for reaching lesbian, gay and bisexual young adults; our results also provide new information for program implementation (e.g., concerns). Additional research is needed to determine how to best address these concerns, identify effective modes for implementation (including receipt of results and follow-up with treatment for positive tests) and evaluate the impact of this novel strategy on increasing access to STD screening among sexual minority young adults.
Acknowledgments
Support for this project was provided by the National Cancer Institute at the National Institutes of Health (P30CA016058).
Footnotes
“Unmarried” includes never-married, divorced, separated and widowed individuals.
Contributor Information
Annie-Laurie McRee, Assistant professor, Division of Health Behavior and Health Promotion, Ohio State University College of Public Health, Columbus.
Allahna Esber, Doctoral candidate, Division of Epidemiology, Ohio State University College of Public Health, Columbus.
Paul L. Reiter, Assistant professor, Division of Cancer Prevention and Control, Ohio State University College of Medicine
References
- 1.Centers for Disease Control and Prevention (CDC) Sexually Transmitted Disease Surveillance 2012; Atlanta: CDC; 2013. [Google Scholar]
- 2.Miller WC, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Journal of the American Medical Association. 2004;291(18):2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 3.Risser WL, et al. The epidemiology of sexually transmitted infections in adolescents. Seminars in Pediatric Infectious Diseases. 2005;16(3):160–167. doi: 10.1053/j.spid.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Scholes D, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. New England Journal of Medicine. 1996;334(21):1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 5.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesson HW, et al. A brief review of the estimated economic burden of sexually transmitted diseases in the United States: inflation-adjusted updates of previously published cost studies. Sexually Transmitted Diseases. 2011;38(10):889–891. doi: 10.1097/OLQ.0b013e318223be77. [DOI] [PubMed] [Google Scholar]
- 7.Fortenberry JD, et al. Relationships of stigma and shame to gonorrhea and HIV screening. American Journal of Public Health. 2002;92(3):378–381. doi: 10.2105/ajph.92.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham SD, et al. Relationships between perceived STD-related stigma, STD-related shame and STD screening among a household sample of adolescents. Perspectives on Sexual and Reproductive Health. 2009;41(4):225–230. doi: 10.1363/4122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satterwhite CL, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sexually Transmitted Diseases. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 11.Mojola SA, Everett B. STD and HIV risk factors among U S young adults: variations by gender, race, ethnicity and sexual orientation. Perspectives on Sexual and Reproductive Health. 2012;44(2):125–133. doi: 10.1363/4412512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindley LL, et al. STDs among sexually active female college students: Does sexual orientation make a difference? Perspectives on Sexual and Reproductive Health. 2008;40(4):212–217. doi: 10.1363/4021208. [DOI] [PubMed] [Google Scholar]
- 13.Charlton BM, et al. Reproductive health screening disparities and sexual orientation in a cohort study of U. S adolescent and young adult females. Journal of Adolescent Health. 2011;49(5):505–510. doi: 10.1016/j.jadohealth.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaestle CE, Waller MW. Bacterial STDs and perceived risk among sexual minority young adults. Perspectives on Sexual and Reproductive Health. 2011;43(3):158–163. doi: 10.1363/4315811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrazzo JM, Coffey P, Bingham A. Sexual practices, risk perception and knowledge of sexually transmitted disease risk among lesbian and bisexual women. Perspectives on Sexual and Reproductive Health. 2005;37(1):6–12. doi: 10.1363/psrh.37.006.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey JV, et al. Sexually transmitted infections in women who have sex with women. Sexually Transmitted Infections. 2004;80(3):244–246. doi: 10.1136/sti.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra A, et al. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. National Health Statistics Reports. 2011;(36) [PubMed] [Google Scholar]
- 18.CDC. Sexually Transmitted Diseases Treatment Guidelines. Atlanta: CDC; 2010. [Google Scholar]
- 19.U.S. Department of Health and Human Services. [accessed June 16, 2014];Healthy People 2020 Objectives: Sexually Transmitted Diseases. 2014 < http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=37>.
- 20.Fiscus LC, Ford CA, Miller WC. Infrequency of sexually transmitted disease screening among sexually experienced U. S female adolescents. Perspectives on Sexual and Reproductive Health. 2004;36(6):233–238. doi: 10.1363/psrh.36.233.04. [DOI] [PubMed] [Google Scholar]
- 21.Ku L, et al. Risk behaviors, medical care, and chlamydial infection among young men in the United States. American Journal of Public Health. 2002;92(7):1140–1143. doi: 10.2105/ajph.92.7.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerker BD, Mostashari F, Thorpe L. Health care access and utilization among women who have sex with women: sexual behavior and identity. Journal of Urban Health. 2006;83(5):970–979. doi: 10.1007/s11524-006-9096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein KT, et al. Same-sex attraction disclosure to health care providers among New York City men who have sex with men: implications for HIV testing approaches. Archives of Internal Medicine. 2008;168(13):1458–1464. doi: 10.1001/archinte.168.13.1458. [DOI] [PubMed] [Google Scholar]
- 24.Coker TR, Austin SB, Schuster MA. The health and health care of lesbian, gay, and bisexual adolescents. Annual Review of Public Health. 2010;31:457–477. doi: 10.1146/annurev.publhealth.012809.103636. [DOI] [PubMed] [Google Scholar]
- 25.Marrazzo JM, et al. Genital human papillomavirus infection in women who have sex with women. Journal of Infectious Diseases. 1998;178(6):1604–1609. doi: 10.1086/314494. [DOI] [PubMed] [Google Scholar]
- 26.Tilson EC, et al. Barriers to asymptomatic screening and other STD services for adolescents and young adults: focus group discussions. [accessed July 7, 2014];BMC Public Health. 2004 4 doi: 10.1186/1471-2458-4-21. Art. 21 < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC436061/#>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimiaga MJ, et al. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sexually Transmitted Diseases. 2007;34(2):113–119. doi: 10.1097/01.olq.0000225327.13214.bf. [DOI] [PubMed] [Google Scholar]
- 28.Falk L, et al. Sampling for Chlamydia trachomatis infection—a comparison of vaginal, first-catch urine, combined vaginal and first-catch urine and endocervical sampling. International Journal of STD & AIDS. 2010;21(4):283–287. doi: 10.1258/ijsa.2009.009440. [DOI] [PubMed] [Google Scholar]
- 29.van der Helm JJ, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sexually Transmitted Diseases. 2009;36(8):493–497. doi: 10.1097/OLQ.0b013e3181a44b8c. [DOI] [PubMed] [Google Scholar]
- 30.Page C, Mounsey A, Rowland K. PURLs: Is self-swabbing for STIs a good idea? Journal of Family Practice. 2013;62(11):651–653. [PMC free article] [PubMed] [Google Scholar]
- 31.Fielder RL, Carey KB, Carey MP. Acceptability of sexually transmitted infection testing using self-collected vaginal swabs among college women. Journal of American College Health. 2013;61(1):46–53. doi: 10.1080/07448481.2012.750610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huppert JS, et al. Acceptability of self-testing for trichomoniasis increases with experience. Sexually Transmitted Infections. 2011;87(6):494–500. doi: 10.1136/sextrans-2011-050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marrazzo JM, Scholes D. Acceptability of urine-based screening for Chlamydia trachomatis in asymptomatic young men: a systematic review. Sexually Transmitted Diseases. 2008;35(11 Suppl):S28–S33. doi: 10.1097/OLQ.0b013e31816938ca. [DOI] [PubMed] [Google Scholar]
- 34.Wayal S, et al. Self-sampling for oropharyngeal and rectal specimens to screen for sexually transmitted infections: acceptability among men who have sex with men. Sexually Transmitted Infections. 2009;85(1):60–64. doi: 10.1136/sti.2008.032193. [DOI] [PubMed] [Google Scholar]
- 35.Gaydos CA, et al. Can e-technology through the Internet be used as a new tool to address the Chlamydia trachomatis epidemic by home sampling and vaginal swabs? Sexually Transmitted Diseases. 2009;36(9):577–580. doi: 10.1097/OLQ.0b013e3181a7482f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippman SA, et al. Home-based self-sampling and self-testing for sexually transmitted infections: acceptable and feasible alternatives to provider-based screening in low-income women in São Paulo, Brazil. Sexually Transmitted Diseases. 2007;34(7):421–428. doi: 10.1097/01.olq.0000245958.34961.27. [DOI] [PubMed] [Google Scholar]
- 37.Llewellyn C, et al. Are home sampling kits for sexually transmitted infections acceptable among men who have sex with men? Journal of Health Services Research & Policy. 2009;14(1):35–43. doi: 10.1258/jhsrp.2008.007065. [DOI] [PubMed] [Google Scholar]
- 38.Graseck AS, et al. Home screening compared with clinic-based screening for sexually transmitted infections. Obstetrics & Gynecology. 2010;115(4):745–752. doi: 10.1097/AOG.0b013e3181d4450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter PL, et al. HPV vaccination among young adult gay and bisexual men in the United States. American Journal of Public Health. 2014;105(1):96–102. doi: 10.2105/AJPH.2014.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McRee AL, et al. HPV vaccination among lesbian and bisexual women: findings from a national survey of young adults. Vaccine. 2014;32(37):4736–4742. doi: 10.1016/j.vaccine.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HarrisInteractive. [accessed Dec. 20, 2013];LGBTpanel. 2014 < http://www.harrisinteractive.com/MethodsTools/DataCollection/SpecialtyPanelsPanelDevelopment/LGBTPanel.aspx>.
- 42.Harris Interactive. [accessed Dec. 20, 2013];Harris poll online panel. 2014 < http://www.harrisinteractive.com/MethodsTools/DataCollection/HarrisPollOnlinePanel.aspx>.
- 43.Jones HE, et al. Women’s preferences for testing and management of sexually transmitted infections among low-income New York City family planning clients. International Journal of STD & AIDS. 2013;24(6):455–460. doi: 10.1177/0956462412473888. [DOI] [PubMed] [Google Scholar]
- 44.Jamil MS, et al. Home-based chlamydia and gonorrhea screening: a systematic review of strategies and outcomes. [accessed Nov. 5, 2014];BMC Public Health. 2013 13 doi: 10.1186/1471-2458-13-189. Art. 189, < http://www.biomedcentral.com/1471-2458/13/189>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford CA, et al. Perceived risk of chlamydial and gonococcal infection among sexually experienced young adults in the United States. Perspectives on Sexual and Reproductive Health. 2004;36(6):258–264. doi: 10.1363/psrh.36.258.04. [DOI] [PubMed] [Google Scholar]
- 46.Jeffries WL., 4th HIV testing among bisexual men in the United States. AIDS Education and Prevention. 2010;22(4):356–370. doi: 10.1521/aeap.2010.22.4.356. [DOI] [PubMed] [Google Scholar]
- 47.Collins SR, Garber T, Robertson R. Realizing Health Reform’s Potential: How the Affordable Care Act Is Helping Young Adults Stay Covered. New York: Commonwealth Fund; 2011. [PubMed] [Google Scholar]
- 48.Owens SL, et al. Utilizing the Internet to test for sexually transmitted infections: results of a survey and accuracy testing. Sexually Transmitted Infections. 2010;86(2):112–116. doi: 10.1136/sti.2009.037226. [DOI] [PubMed] [Google Scholar]
- 49.English A, et al. Confidentiality for Individuals Insured as Dependents: A Review of State Laws and Policies. New York: Guttmacher Institute and Public Health Solutions; 2012. [accessed Oct. 31, 2014]. < http://www.guttmacher.org/pubs/confidentiality-review.pdf>. [Google Scholar]
- 50.Frerich EA, et al. Health care reform and young adults’ access to sexual health care: an exploration of potential confidentiality implications of the Affordable Care Act. American Journal of Public Health. 2012;102(10):1818–1821. doi: 10.2105/AJPH.2012.300857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman AL, Bloodgood B. Exploring the feasibility of alternative STD-testing venues and results delivery channels for a national screening campaign. Health Promotion Practice. 2013;14(1):96–104. doi: 10.1177/1524839911404226. [DOI] [PubMed] [Google Scholar]
- 52.Gaydos CA, et al. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sexually Transmitted Diseases. 2006;33(7):451–457. doi: 10.1097/01.olq.0000200497.14326.fb. [DOI] [PubMed] [Google Scholar]
- 53.Chai SJ, et al. Internet-based screening for sexually transmitted infections to reach nonclinic populations in the community: risk factors for infection in men. Sexually Transmitted Diseases. 2010;37(12):756–763. doi: 10.1097/OLQ.0b013e3181e3d771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. American Journal of Health Behavior. 2003;27(Suppl 3):S227–S232. doi: 10.5993/ajhb.27.1.s3.6. [DOI] [PubMed] [Google Scholar]
- 55.Ford CA, Viadro CI, Miller WC. Testing for chlamydial and gonorrheal infections outside of clinic settings: a summary of the literature. Sexually Transmitted Diseases. 2004;31(1):38–51. doi: 10.1097/01.OLQ.0000105117.77684.B9. [DOI] [PubMed] [Google Scholar]
- 56.Klein JD, et al. Developing quality measures for adolescent care: validity of adolescents’ self-reported receipt of preventive services. Health Services Research. 1999;34(1 Pt 2):391–404. [PMC free article] [PubMed] [Google Scholar]
- 57.Sieving RE, et al. Development of adolescent self-report measures from the National Longitudinal Study of Adolescent Health. Journal of Adolescent Health. 2001;28(1):73–81. doi: 10.1016/s1054-139x(00)00155-5. [DOI] [PubMed] [Google Scholar]
- 58.Kreuter F, Presser S, Tourangeau R. Social desirability bias in CATI, IVR, and Web surveys: the effects of mode and question sensitivity. Public Opinion Quarterly. 2008;72(5):847–865. [Google Scholar]
- 59.McEachan RRC, et al. Prospective prediction of health-related behaviours with the theory of planned behavior: a meta-analysis. Health Psychology Review. 2011;5(2):97–144. [Google Scholar]