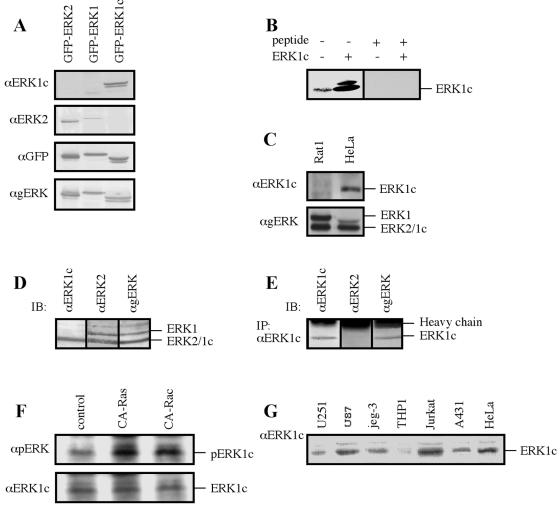
FIG. 4.
Characterization of the endogenous ERK1c protein. (A) Testing the specificity of the anti-ERK1c Ab. HEK-293 cells were transfected with the indicated constructs, and extracts (containing cytosolic and nuclear proteins; 50 μg) were immunoblotted with four different Abs: anti-ERK1c Ab (αERK1c), anti-ERK2 Ab (αERK2), anti-GFP Ab (αGFP), or anti-ERK1/2 Ab (αgERK). (B) Competing the anti-ERK1c Ab with the antigenic peptide. HeLa cell extract (containing cytosolic and nuclear proteins; 50 μg) from nonstimulated HeLa cells transfected with ERK1c (+) or not transfected (−) were subjected to immunoblotting with anti-ERK1c Ab (ERK1c) in the absence (−) or presence (+) of the antigenic peptide (25 μg/ml). (C) ERK1c is not expressed in rat cells. HeLa and Rat1 cell extracts (50 μg) were loaded on SDS-polyacrylamide gels and immunoblotted with the indicated Abs. (D) ERK1c migrates together with ERK2 on SDS-polyacrylamide gels. HeLa cell extracts were loaded on one lane (100 μg) of the SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. After the transfer, the membrane was cut into three pieces, and each piece was immunoblotted (IB) with a different Ab as indicated. (E) Immunoprecipitation of ERK1c from HeLa cells. An anti-ERK1c Ab was used to immunoprecipitate (IP) ERK1c from HeLa cell extract (500 μg), followed by immunoblotting (IB) with the indicated Abs. (F) ERK1c is phosphorylated on its Thr and Tyr residues upon activation. HeLa cells were transfected with constitutively activated Ras (CA-Ras), constitutively activated Rac (CA-Rac), or a vector control. ERK1c was immunoprecipitated with the anti-ERK1c Ab and immunoblotted with anti-pERK and anti-ERK1c Abs. (G) ERK1c expression in several human tumor cell lines. Cell lysate (50 μg) from a nonstimulated human glioblastoma brain tumor cell line, U-251 MG, U87 PTEN-deficient glioma cells, human choriocarcinoma cell line jeg-3, a natural human monocytic cell line (THP1), Jurkat T lymphocytes, A431 epidermal carcinoma cells, or HeLa cells were subjected to immunoblotting with anti-ERK1c Ab. The amounts of ERK1 and ERK2 detected in this experiment by anti-ERK Ab were similar in all cells. The experiments were reproduced three times.