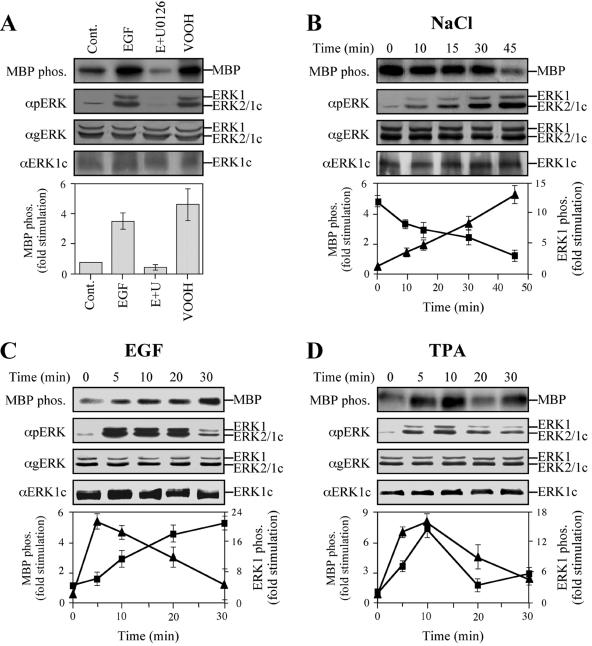
FIG. 5.
(A) Kinetics of ERK1c activation. Stimulation and inhibition of MBP phosphorylation (MBP phos.) by ERK1c are shown. HeLa cells were serum starved for 16 h and then stimulated with either EGF (50 ng/ml, 10 min), EGF plus U0126 (E+U) (15-min pretreatment with 5 μM U0126, followed by 10 min of EGF [50 ng/ml]), or VOOH (100 μM Na3VO4 and 200 μM H2O2, 15 min) or left untreated as a control (Cont.). After harvesting, extracts were either immunoprecipitated with anti-ERK1c Ab (αERK1c) and subjected to an in vitro MBP phosphorylation (MBP phos.), or they were immunoblotted with anti-ERK1c, anti-pERK, or anti-gERK Ab. The bar graph shows the means ± standard errors (error bars) from three different experiments. (B) Time course of ERK1c activation by osmotic shock. HeLa cells were serum starved for 16 h and then stimulated with 0.7 M NaCl for the indicated times. ERK1c activity was examined and immunoblotting was performed as described above for panel A. Squares represent ERK1c activity, while the triangles represent the phosphorylation (phos.) of ERK1 and ERK2. The results in the graph show the means ± standard errors from three different experiments. (C and D) Time course of ERK1c activation by EGF and TPA. HeLa cells were treated as described above for panel B, except that EGF (50 ng/ml) or TPA (250 nM) was used for stimulation.