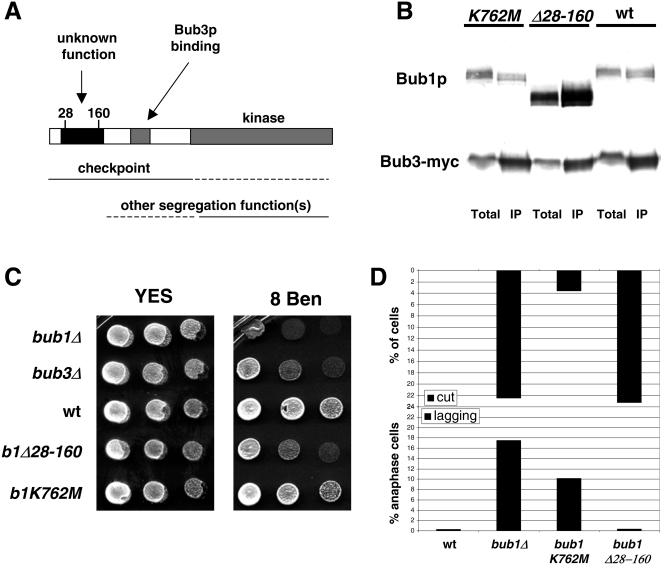
FIG. 7.
The conserved N-terminal domain of Bub1p plays a kinetochore targeting role that is crucial for spindle checkpoint function, but not for Bub3p binding or the prevention of lagging chromosomes. (A) Schematic model of the conserved domains of Bub1p and their likely functions, indicating the position of the deleted region in the N-terminal domain. (B) Bub3-Myc immunoprecipitates were immunoblotted and shown to contain both wild type and mutant Bub1Δ28-160p. No clear effect on this Bub protein interaction was detected. Note that in this anti-Bub1 immunoblot it appears that the Bub1Δ28-160 protein is more abundant than wild-type Bub1p, both in the crude extract (Total) and the anti-Bub3 immunoprecipitate (IP). While this was reproducible, we do not believe it to be the case. In fact, our Bub1 antibody recognized the mutant protein better than wild-type Bub1p (see Fig. S2B in the supplemental material). (C) The indicated strains were tested for benomyl sensitivity. Images were taken after growth at 30°C for 4 days. (D) Quantitation of checkpoint and chromosome segregation defects in different bub1 alleles. Quantitation of the Cut phenotype observed in different bub1 nda3 strains after 6 h at 18°C was plotted as a percentage of the total population (see Fig. S2C in the supplemental material for supporting images). Lagging chromosomes were quantitated after fixing cells and staining them with 4′,6′-diamidino-2-phenylindole and antitubulin antibodies, and results are plotted as the percentage of anaphase cells displaying them (7).