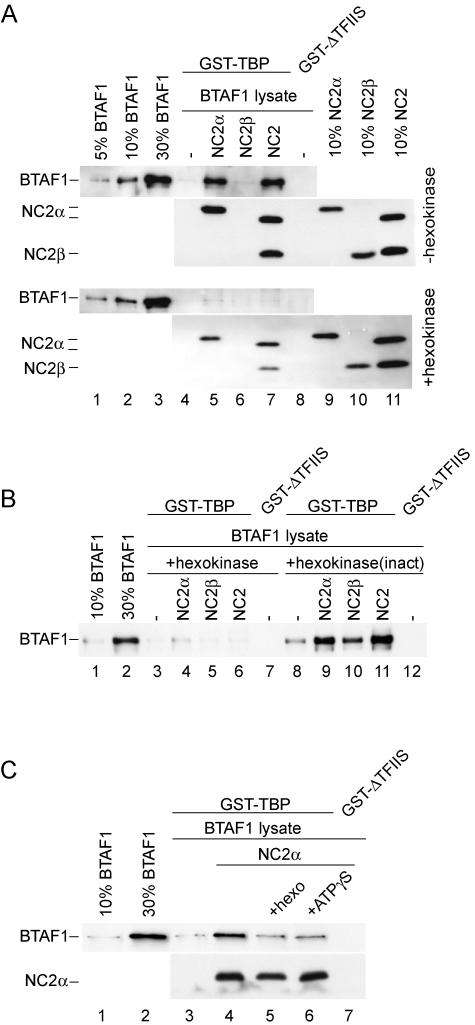
FIG. 4.
NC2α stimulates the interaction of BTAF1 with TBP in an ATP-dependent manner. (A) RK13 cell lysate (1 μl) containing overexpressed BTAF1 was incubated with glutathione-agarose beads coated with GST-TBP (lanes 4 to 7) or GST-ΔTFIIS (lane 8) in the absence (lanes 4 and 8) or presence of 6.5 pmol of hisNC2α (lane 5), hisNC2β (lane 6), and NC2(α/hisβ) (lane 7) as described in Materials and Methods. Lanes 4 to 8 in the bottom panel contained 10 U of hexokinase and 10 mM glucose. (B) Hexokinase activity is necessary to abolish NC2α-mediated binding of BTAF1 and TBP. RK13 cell lysate (1 μl) containing overexpressed BTAF1 was incubated with glutathione-agarose beads coated with GST-TBP (lanes 3 to 6 and 8 to 11) or GST-ΔTFIIS (lanes 7 and 12) in the absence (lanes 3, 7, 8, and 12) or presence of 6.5 pmol of hisNC2α (lanes 4 and 9), hisNC2β (lanes 5 and 10), and NC2(α/hisβ) (lanes 6 and 11). Lanes 3 to 7 contained 10 U of hexokinase and 10 mM glucose. Lanes 8 to 12 contained 10 U of hexokinase inactivated by boiling for 3 min [hexokinase(inact)] and 10 mM glucose. (C) Excess ATP analogue inhibits NC2α-stimulated BTAF1-TBP interaction. RK13 cell lysate (1 μl) containing overexpressed BTAF1 was incubated with glutathione-agarose beads coated with GST-TBP (lanes 3 to 6) or GST-ΔTFIIS (lane 7) in the absence (lanes 3 and 7) or presence of 6.5 pmol of hisNC2α (lanes 4 to 6). Lane 5 contained 4 U of hexokinase (hexo) and 5 mM glucose. Lane 6 contained 2 mM ATPγS. All reaction mixtures additionally contained 5 mM magnesium chloride.