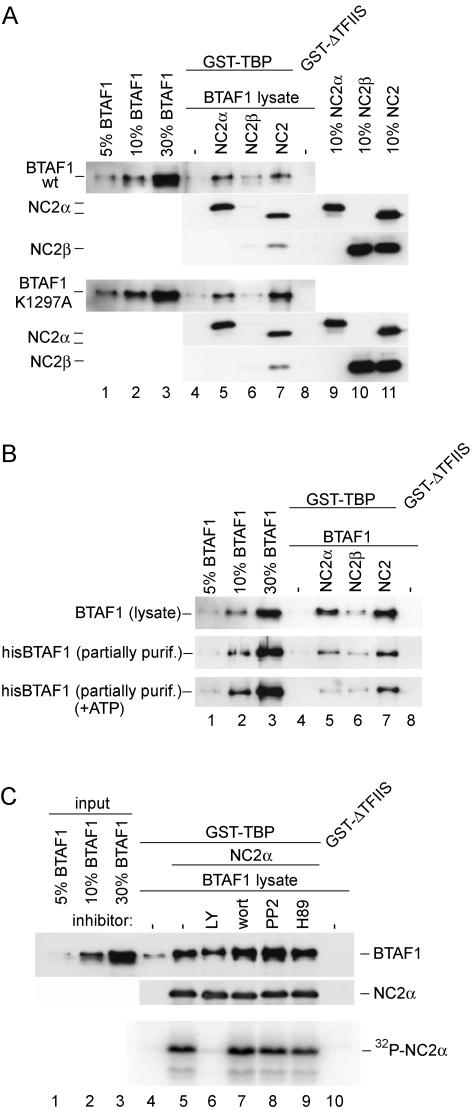
FIG. 5.
ATP hydrolysis by BTAF1 or NC2α phosphorylation is not required for its binding to TBP in the presence of NC2α. (A) The ATPase-deficient mutant of BTAF1 binds to TBP in the presence of NC2α. Reactions were performed as described for Fig. 4A. BTAF1-containing HeLa cell lysates were diluted with the lysate of cells infected with T7 polymerase-expressing virus to obtain equal concentrations of wild-type and mutant BTAF1. (B) ATP does not increase BTAF1-TBP interaction in the presence of NC2α. Reactions were performed as described for Fig. 4A, except that the reaction mixtures shown in the top panel received 0.5 μl of BTAF1-enriched RK13 cell lysate, and those shown in the middle and bottom panels received 0.5 μl of partially purified (purif.) hisBTAF1. Reaction mixtures shown in the bottom panel also contained 1 mM ATP, 20 U of creatine phos-phokinase, and 10 mM creatine phosphate. (C) NC2α phosphorylation is not required for BTAF1-TBP interaction. The reaction was performed as described for panel A, except that 10 times more GST-TBP, hisNC2α, and BTAF1-containing lysate was added. Twenty microcuries of [32P]γATP and 5 mM MgCl2 were included in all the reactions. Lane 6 included 50 μM LY194002 (LY), lane 7 contained 10 μM wortmannin (wort), lane 8 contained 50 μM PP2, and lane 9 contained 50 μM H89. The top panel represents the immunoblotting experiment, whereas the bottom panel represents the autoradiogram.