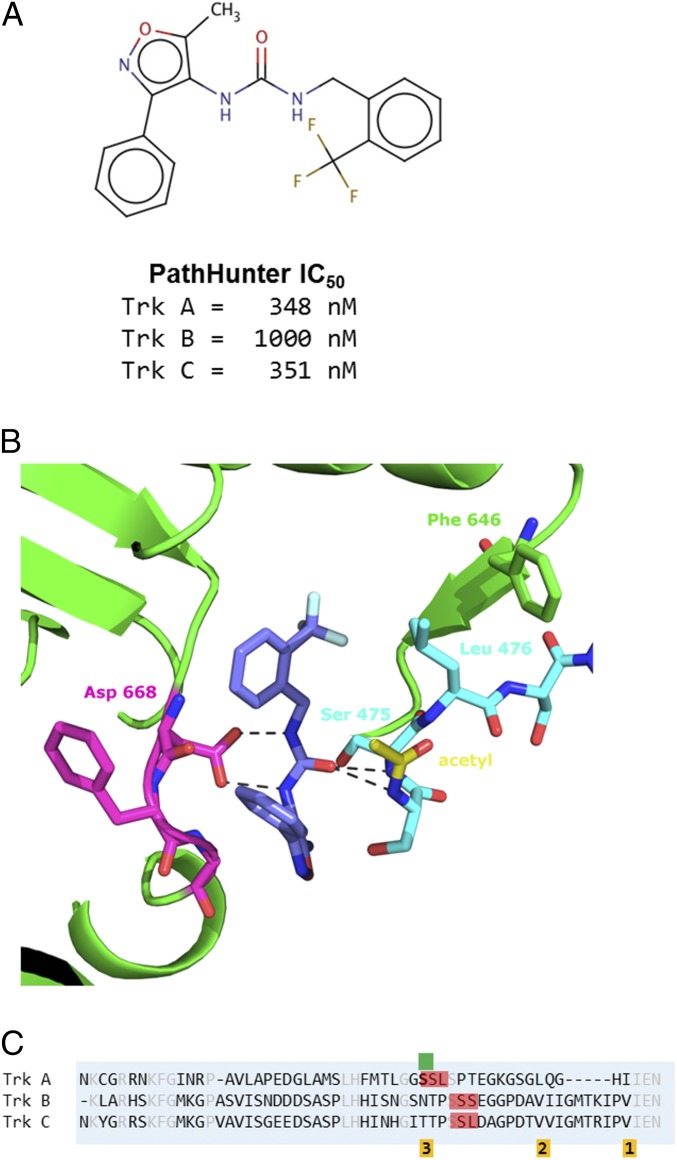
Fig. 5.
Structure of nonselective compound 4 binding in mode 3. (A) Cell-based, PathHunter IC50 values for compound 4 against Trks A and Trk C, showing that the compound is not TrkA-selective. (B) Zoom-in view of compound 4 binding. The central urea is positioned to make a bidentate interaction with the carboxyl oxygens of Asp-668. Ser-475 sits above the fluorophenyl, which is referred to as mode 3. A second JM residue, Leu-476, also occupies the pocket, forcing Phe-646 of the kinase domain to displaced further. The acetyl modification on the amino terminus of the expressed protein is shown with carbons in yellow. (C) Sequence alignment of the JM regions of Trks A, B, and C. The SSL and SSS sequences are indicated in red. The residues involved in packing against the hydrophobic pocket of modes 1, 2, and 3 are indicated below the sequence in orange. Serine 474, the first residue of the JM-kinase construct is indicated by the green marker.