Abstract
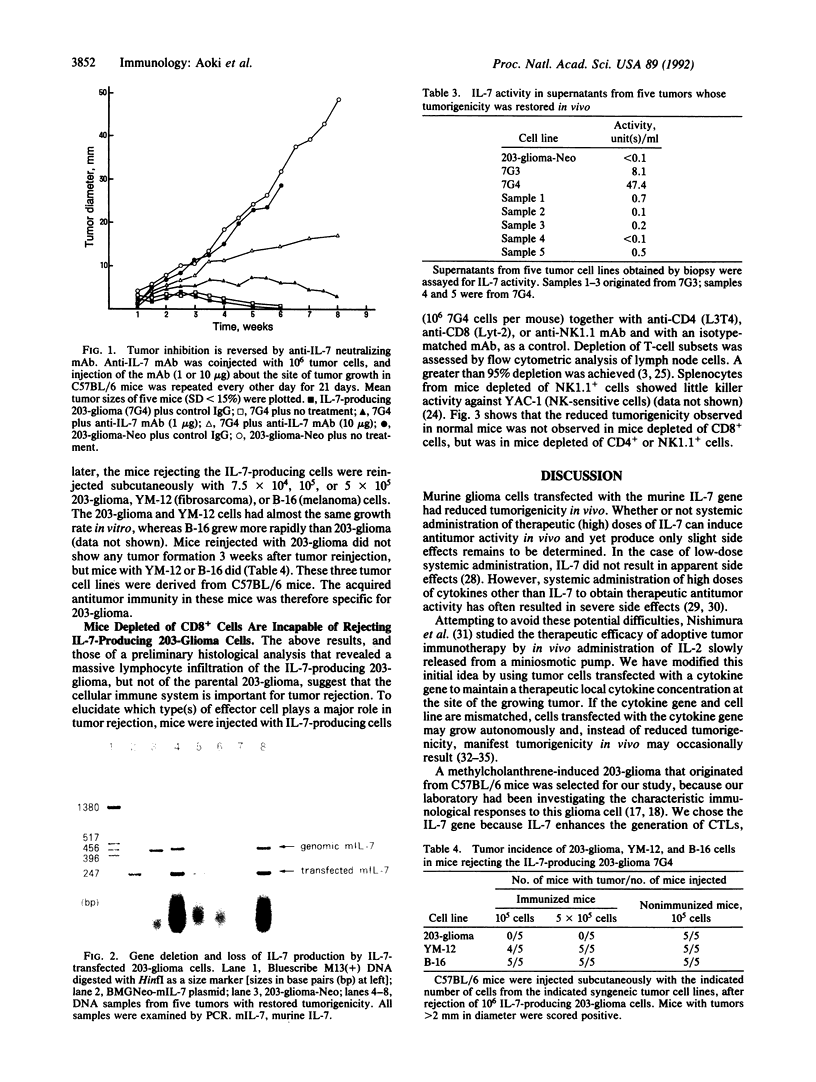
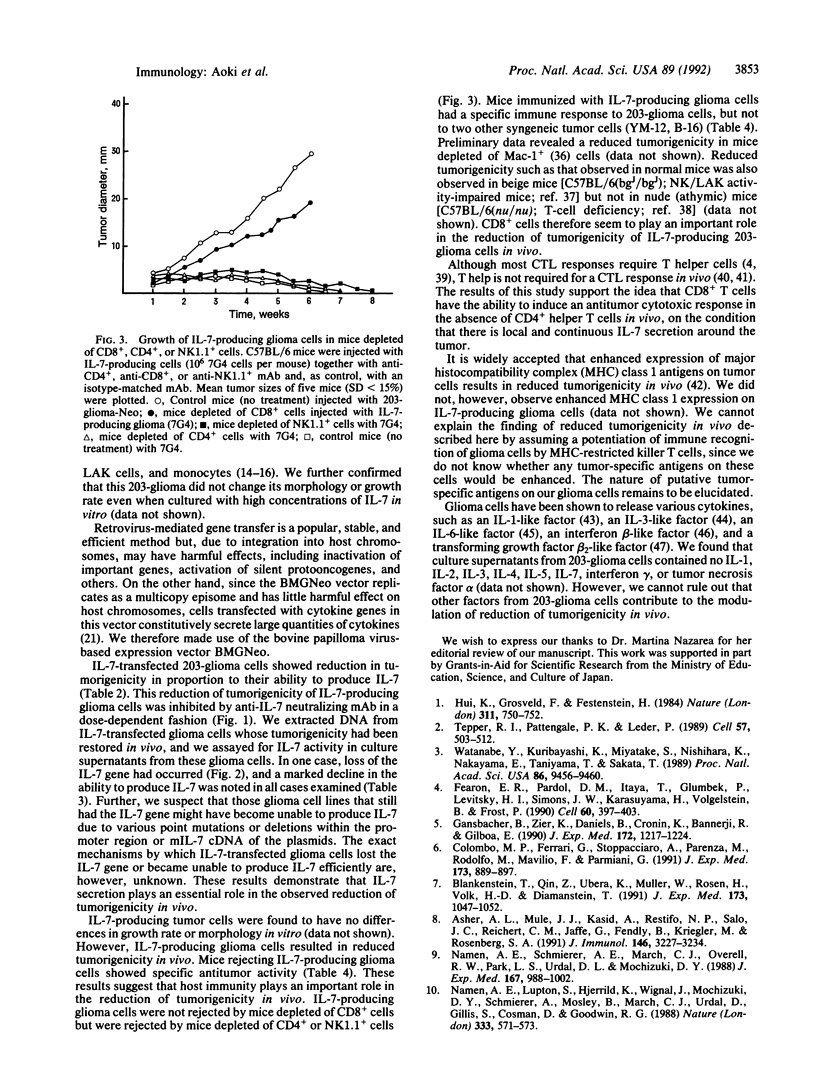
We have examined the immunoregulatory effect of local and continuous secretion of interleukin 7 (IL-7) from murine glioma cells (203-glioma) engineered by murine IL-7 gene transfection. Secretion of IL-7 from glioma cells did not result in morphology or growth rate changes but did reduce tumorigenicity in vivo in proportion to the amount of IL-7 produced. This reduction in tumorigenicity could be reversed in a dose-dependent fashion by injection of anti-IL-7 neutralizing monoclonal antibody at the tumor site. Mice immunized with IL-7-producing glioma cells showed a specific immune response to 203-glioma but not to two other syngeneic cell lines (B-16, a melanoma, and YM-12, a fibrosarcoma). IL-7-producing glioma cells were not rejected in mice depleted of CD8+ cells but were rejected in mice depleted of CD4+ or NK1.1+ cells. These results suggest that CD8+ T cells may play an important role in tumor rejection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Sassenfeld H. M., Widmer M. B. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990 Aug 1;172(2):577–587. doi: 10.1084/jem.172.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson M. R., Tough T. W., Ziegler S. F., Grabstein K. H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991 Apr 1;173(4):923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Kikuchi H., Miyatake S., Oda Y., Iwasaki K., Yamasaki T., Kinashi T., Honjo T. Interleukin 5 enhances interleukin 2-mediated lymphokine-activated killer activity. J Exp Med. 1989 Aug 1;170(2):583–588. doi: 10.1084/jem.170.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Tashiro K., Miyatake S., Nakano T., Oda Y., Kikuchi H., Honjo T. Expression of the RAG-2 gene in murine central nervous system tumor cell lines. Biochem Biophys Res Commun. 1991 Nov 27;181(1):151–158. doi: 10.1016/s0006-291x(05)81394-4. [DOI] [PubMed] [Google Scholar]
- Asher A. L., Mulé J. J., Kasid A., Restifo N. P., Salo J. C., Reichert C. M., Jaffe G., Fendly B., Kriegler M., Rosenberg S. A. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991 May 1;146(9):3227–3234. [PMC free article] [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Uberla K., Müller W., Rosen H., Volk H. D., Diamantstein T. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991 May 1;173(5):1047–1052. doi: 10.1084/jem.173.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Chazen G. D., Pereira G. M., LeGros G., Gillis S., Shevach E. M. Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5923–5927. doi: 10.1073/pnas.86.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Martin G., Qin S., Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986 Sep 11;323(6084):164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- Colombo M. P., Ferrari G., Stoppacciaro A., Parenza M., Rodolfo M., Mavilio F., Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991 Apr 1;173(4):889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985 Dec;135(6):4044–4047. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Lupton S., Schmierer A., Hjerrild K. J., Jerzy R., Clevenger W., Gillis S., Cosman D., Namen A. E. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Fiers W., North R. J. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988 Mar 1;167(3):1067–1085. doi: 10.1084/jem.167.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K., Grosveld F., Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984 Oct 25;311(5988):750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Keene J. A., Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982 Mar 1;155(3):768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo G. C., Dumont F. J., Tutt M., Hackett J., Jr, Kumar V. The NK-1.1(-) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986 Dec 15;137(12):3742–3747. [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Larsson I., Landström L. E., Larner E., Lundgren E., Miörner H., Strannegård L. Interferon production in glia and glioma cell lines. Infect Immun. 1978 Dec;22(3):786–789. doi: 10.1128/iai.22.3.786-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. H., Miller R. E. Induction of murine lymphokine-activated killer cells by recombinant IL-7. J Immunol. 1990 Sep 15;145(6):1983–1990. [PubMed] [Google Scholar]
- Morrissey P. J., Conlon P., Charrier K., Braddy S., Alpert A., Williams D., Namen A. E., Mochizuki D. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991 Jul 15;147(2):561–568. [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Togashi Y., Goto M., Yagi H., Uchiyama Y., Hashimoto Y. Augmentation of the therapeutic efficacy of adoptive tumor immunotherapy by in vivo administration of slowly released recombinant interleukin 2. Cancer Immunol Immunother. 1986;21(1):12–18. doi: 10.1007/BF00199371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W. P., Schilham M. W., Kündig T. M., Sambhara S. R., Narendran A., Arabian A., Wakeham A., Paige C. J., Zinkernagel R. M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991 Sep 12;353(6340):180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Scala G., Quinto I., Ruocco M. R., Arcucci A., Mallardo M., Caretto P., Forni G., Venuta S. Expression of an exogenous interleukin 6 gene in human Epstein Barr virus B cells confers growth advantage and in vivo tumorigenicity. J Exp Med. 1990 Jul 1;172(1):61–68. doi: 10.1084/jem.172.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Tohyama K., Lee K. H., Tashiro K., Kinashi T., Honjo T. Establishment of an interleukin-5-dependent subclone from an interleukin-3-dependent murine hemopoietic progenitor cell line, LyD9, and its malignant transformation by autocrine secretion of interleukin-5. EMBO J. 1990 Jun;9(6):1823–1830. doi: 10.1002/j.1460-2075.1990.tb08307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meir E., Sawamura Y., Diserens A. C., Hamou M. F., de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990 Oct 15;50(20):6683–6688. [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. A., Namen A. E., Goodwin R. G., Armitage R., Cooper M. D. Human IL-7: a novel T cell growth factor. J Immunol. 1989 Dec 1;143(11):3562–3567. [PubMed] [Google Scholar]
- Yamada G., Kitamura Y., Sonoda H., Harada H., Taki S., Mulligan R. C., Osawa H., Diamantstein T., Yokoyama S., Taniguchi T. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987 Sep;6(9):2705–2709. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T., Kikuchi H., Yamashita J., Handa H., Kuwata S., Taguchi M., Namba Y., Hanaoka M. Immunoregulatory effects of interleukin 2 and interferon on syngeneic murine malignant glioma-specific cytotoxic T-lymphocytes. Cancer Res. 1988 Jun 1;48(11):2981–2987. [PubMed] [Google Scholar]
- de Martin R., Haendler B., Hofer-Warbinek R., Gaugitsch H., Wrann M., Schlüsener H., Seifert J. M., Bodmer S., Fontana A., Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987 Dec 1;6(12):3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]