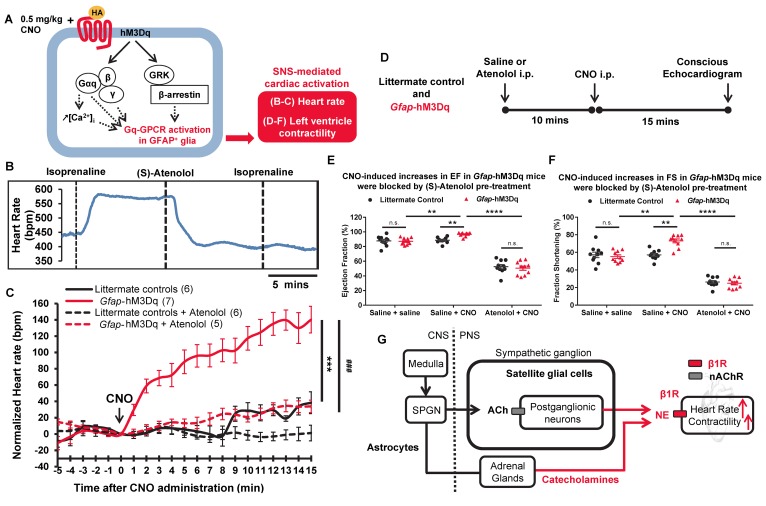
Figure 2. Acute activation of hM3Dq in GFAP+ glia led to robust, beta adrenergic receptor–mediated increases in heart rate and left ventricular contractility in Gfap-hM3Dq mice.
(A) Schematic of in vivo model: CNO-induced hM3Dq activation in GFAP+ glia leads to changes in SNS-driven cardiovascular functions in Gfap-hM3Dq mice. (B) Heart rate recordings showed that 10 mg/kg (S)-atenolol blocked isoprenaline-induced increases in heart rate in C56BL/6J mice in vivo (6 recordings/2 mice). (C) CNO-induced significant increases in heart rate in Gfap-hM3Dq mice over 15 minutes compared with littermate controls (n = 5–7 mice in each group; 2-way ANOVA, ***P < 0.0001, time and genotype interaction between Gfap-hM3Dq and littermate controls), which were blocked by (S)-atenolol in vivo (2-way ANOVA, ###P < 0.0001, time and treatment interaction between two Gfap-hM3Dq groups). Saline or atenolol were injected 10 minutes before CNO. (D) CNO-induced increases in left ventricle functions 15 minutes after CNO injections. Saline or atenolol was injected 10 minutes before CNO. (E) Significant increases in ejection fraction (EF) and (F) fraction shortening (FS) after CNO administration in Gfap-hM3Dq mice (unpaired t test; **P < 0.01 between littermate control and Gfap-hM3Dq mice, also between two Gfap-hM3Dq groups; n = 10 for each genotype), which were blocked by (S)-atenolol pretreatment (unpaired t test; ****P < 0.00001 between the two Gfap-hM3Dq groups; n = 10 for each genotype). (G) Schematic model for potential mechanisms (red lines) underlying CNO-induced cardiac changes in vivo in Gfap-hM3Dq mice. nAChR, nicotinic acetylcholine receptors; SPGN, spinal preganglionic neurons.