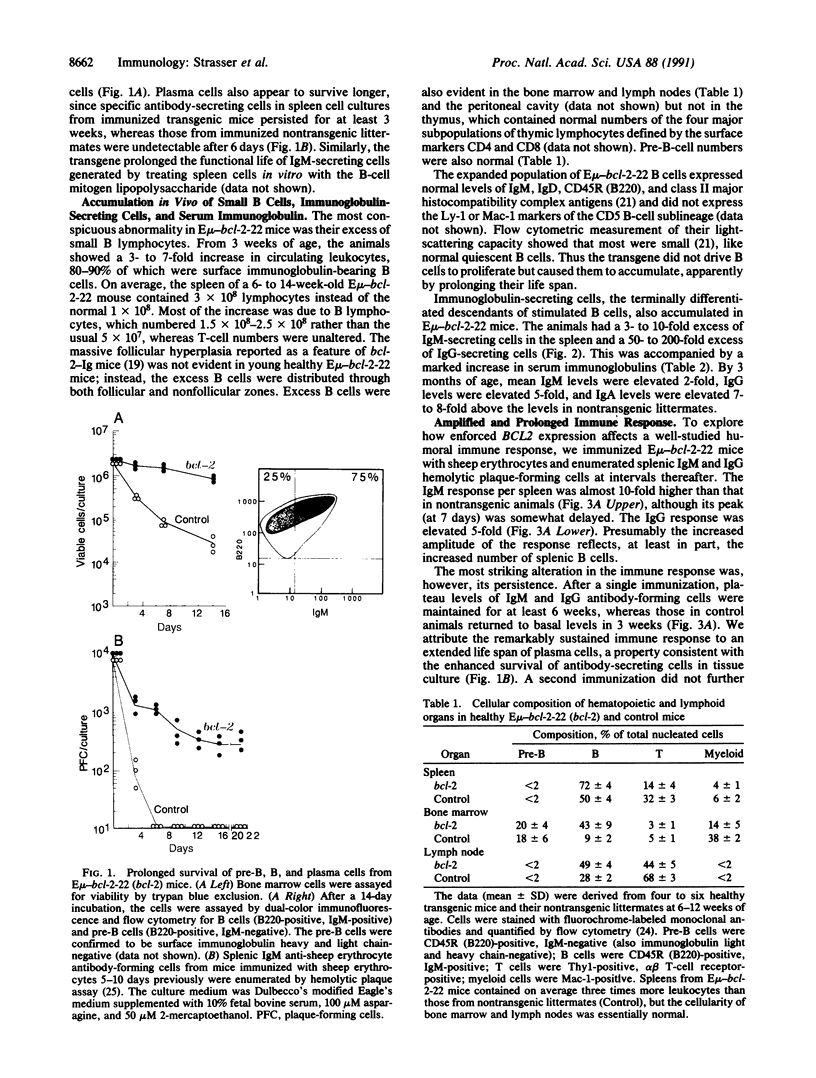
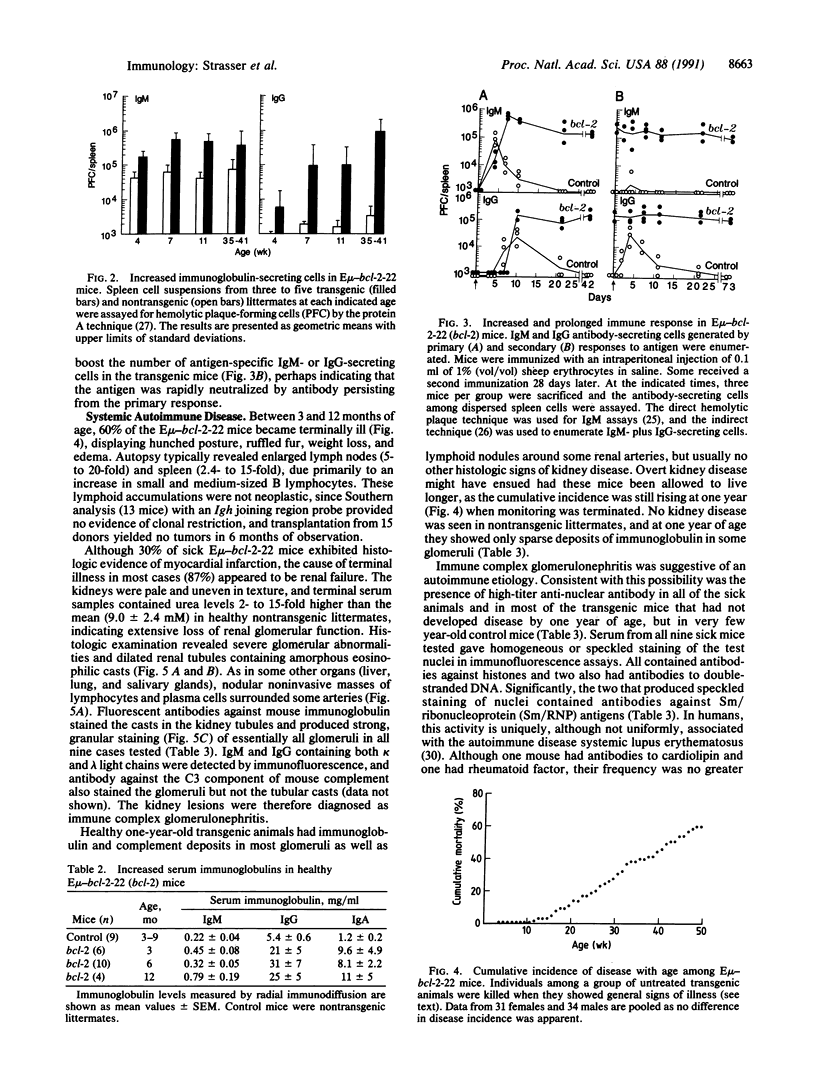
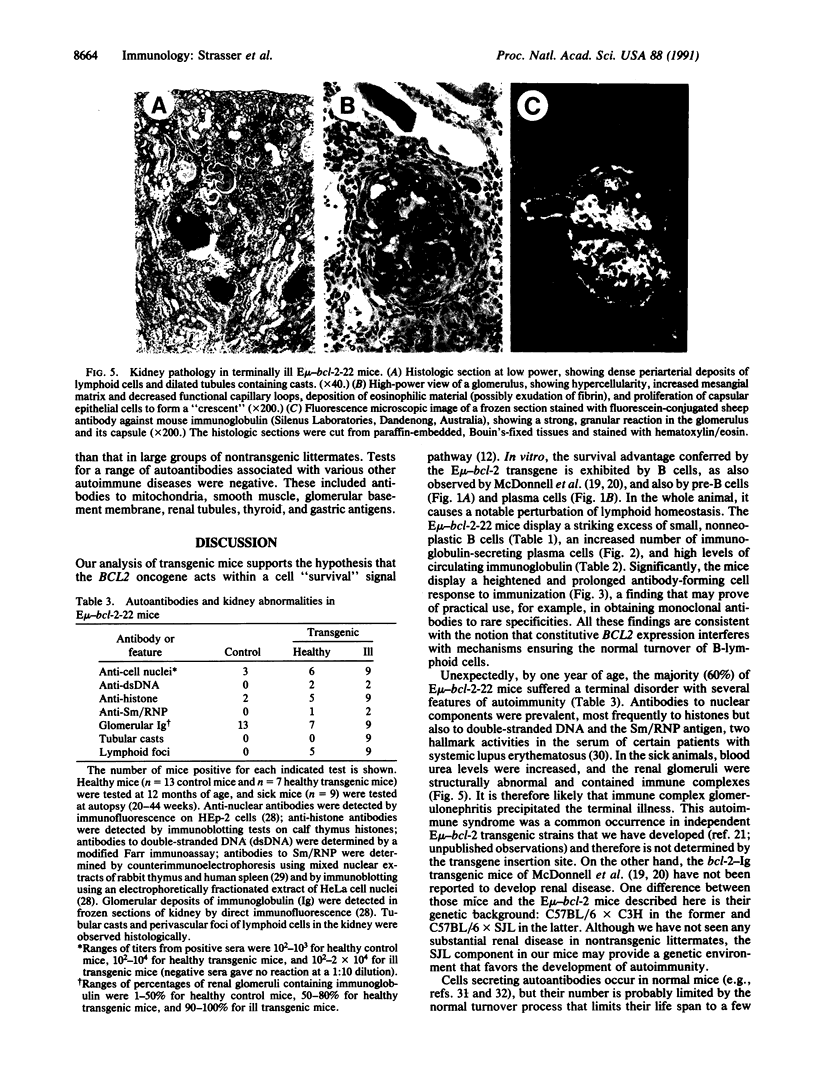
Abstract
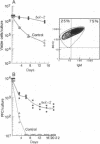
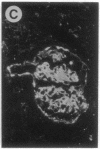
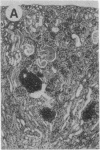
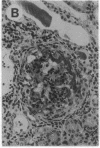
The biological functions of the BCL2 gene were investigated in transgenic mice harboring human BCL2 cDNA under the control of an immunoglobulin heavy chain enhancer (E mu). Mice of a representative transgenic strain, E mu-bcl-2-22, had a great excess of B lymphocytes, immunoglobulin-secreting cells, and serum immunoglobulins, attributable to increased longevity of B-lineage cells. Pre-B and plasma cells as well as B cells exhibited prolonged survival in culture. Immunized animals produced an amplified and protracted antibody response. Within the first year of life, most mice spontaneously produced antibodies to nuclear antigens, and 60% developed kidney disease, diagnosed as immune complex glomerulonephritis. Thus E mu-bcl-2-22 mice constitute a transgenic model for a systemic autoimmune disease resembling the human disorder systemic lupus erythematosus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Tefferi A., Greipp P. R., Kipps T. J., Tsujimoto Y. Preferential linkage of bcl-2 to immunoglobulin light chain gene in chronic lymphocytic leukemia. J Exp Med. 1990 Feb 1;171(2):559–564. doi: 10.1084/jem.171.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B. M., Jacobson J. O. The bcl-2 gene is rearranged in many diffuse B-cell lymphomas. Blood. 1988 Apr;71(4):969–972. [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Graninger W. B., Seto M., Boutain B., Goldman P., Korsmeyer S. J. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987 Nov;80(5):1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata N., Tan E. M. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 1976 May-Jun;19(3):574–580. doi: 10.1002/art.1780190309. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilage L. J., Youngchaiyud U., Whittingham S. Racial differences in antinuclear antibody patterns and clinical manifestations of scleroderma. Arthritis Rheum. 1989 Jan;32(1):54–60. doi: 10.1002/anr.1780320109. [DOI] [PubMed] [Google Scholar]
- Nunez G., Seto M., Seremetis S., Ferrero D., Grignani F., Korsmeyer S. J., Dalla-Favera R. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4589–4593. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Reed J. C., Cuddy M., Haldar S., Croce C., Nowell P., Makover D., Bradley K. BCL2-mediated tumorigenicity of a human T-lymphoid cell line: synergy with MYC and inhibition by BCL2 antisense. Proc Natl Acad Sci U S A. 1990 May;87(10):3660–3664. doi: 10.1073/pnas.87.10.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Haldar S., Cuddy M. P., Croce C., Makover D. Deregulated BCL2 expression enhances growth of a human B cell line. Oncogene. 1989 Sep;4(9):1123–1127. [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Alpers J. D., Croce C. M., Nowell P. C. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. doi: 10.1126/science.3495884. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Radaszkiewicz T., Melchers F. The autoantigen-binding B cell repertoires of normal and of chronically graft-versus-host-diseased mice. J Exp Med. 1987 Jun 1;165(6):1675–1687. doi: 10.1084/jem.165.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum H., Webb E., Adams J. M., Cory S., Harris A. W. N-myc transgene promotes B lymphoid proliferation, elicits lymphomas and reveals cross-regulation with c-myc. EMBO J. 1989 Mar;8(3):749–755. doi: 10.1002/j.1460-2075.1989.tb03435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Vaux D. L., Webb E., Bath M. L., Adams J. M., Cory S. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr Top Microbiol Immunol. 1990;166:175–181. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C. M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2(1):3–7. [PubMed] [Google Scholar]
- Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y. Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989 Nov;4(11):1331–1336. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Dresser D. W., Anderson H. R. Antibody production studied by means of the localized haemolysis in gel (LHG) assay. 3. Mouse cells producing five different classes of antibody. Immunology. 1969 Jul;17(1):93–110. [PMC free article] [PubMed] [Google Scholar]