Abstract
β-Carotene-15,15’-dioxygenase (BCO1) cleaves dietary carotenoids at the central 15,15’ double bond, most notably acting on β-carotene to yield retinal. However, Bco1 disruption also impacts diverse physiological end points independent of dietary carotenoid feeding, including expression of genes controlling androgen metabolism. Using the Bco1−/− mouse model, we sought to probe the effects of Bco1 disruption on testicular steroidogenesis, prostatic androgen signaling, and prostatic proliferation. Male wild-type (WT) and Bco1−/− mice were raised on carotenoid-free AIN-93G diets before euthanasia between 10 and 14 wk of age. Weights of the prostate and seminal vesicles were significantly lower in Bco1−/− than in WT mice (−18% and −29%, respectively). Serum testosterone levels in Bco1−/− mice were significantly reduced by 73%. Bco1 disruption significantly reduced Leydig cell number and decreased testicular mRNA expression of Hsd17b3, suggesting inhibition of testicular testosterone synthesis. Immunofluorescent staining of the androgen receptor (AR) in the dorsolateral prostate lobes of Bco1−/− mice revealed a decrease in AR nuclear localization. Analysis of prostatic morphology suggested decreases in gland size and secretion. These findings were supported by reduced expression of the proliferation marker Ki-67 in Bco1−/− prostates. Expression analysis of 200 prostate cancer- and androgen-related genes suggested that Bco1 loss significantly disrupted prostatic androgen receptor signaling, cell cycle progression, and proliferation. This is the first demonstration that Bco1 disruption lowers murine circulating testosterone levels and thereby reduces prostatic androgen receptor signaling and prostatic cellular proliferation, further supporting the role of this protein in processes more diverse than carotenoid cleavage.
Keywords: Bco1, androgen, prostate, carotenoid oxygenase
experimental evidence demonstrating that β-carotene is a precursor to vitamin A, and therefore as a provitamin, dates to the late 1920s. However, elucidation of the mechanism of action of this conversion remained a mystery until 1965, when two independent reports demonstrated the enzymatic cleavage of β-carotene to retinal using extracts of rat liver and intestine (29, 59). Additional studies over the next several decades determined the characteristics and specificity of this enzyme (42), identified iron as a required cofactor (41), and proposed central cleavage metabolites as the main reaction products (8). Still, the molecular identity of this crucial enzyme remained unknown for 35 yr, until the cloning of the Drosophila ortholog of human β-carotene-15,15’-dioxygenase (BCO1) in 2000 (50). Biochemical characterization of recombinant mammalian enzymes (46, 67), tissue-specific expression in humans (47), and description of mutational events (48) followed in short order. Genomic research has now identified single nucleotide polymorphisms (SNPs) in the human BCO1 gene, which result in altered accumulation of dietary carotenoids, including β-carotene (21, 31, 44).
Meanwhile, carefully controlled studies in mice lacking functional copies of this enzyme demonstrate the importance of BCO1 in carotenoid cleavage. Bco1−/− mice experience dramatic tissue accumulation of β-carotene and are unable to generate vitamin A when fed a diet providing β-carotene as the predominant or only source of vitamin A (33, 49). However, these mice often present additional phenotypes independent of their dietary vitamin A status, including altered lipid metabolism (33), impaired fatty acid, cholesterol, and retinol esterification (19), cardiac dysfunction (43), elevated insulin and leptin levels (23), and changes in expression of genes controlling steroid metabolism (30). A preponderance of evidence now suggests that whereas central cleavage of provitamin A carotenoids to yield retinal represents the main evolutionary function of BCO1, this enzyme likely plays a broader role in an array of physiological processes. Given the demonstrated effect of Bco1 disruption on the expression of genes responsible for steroid metabolism (30) and the importance of steroids in prostatic physiology, we questioned the extent to which alteration in function of this carotenoid oxygenase may alter androgen biology in male mice, independently of its role in carotenoid cleavage. Therefore, in this report, we have assessed the impact of homozygous genomic Bco1 disruption on systemic androgen status and prostatic physiology.
MATERIALS AND METHODS
Mice and study designs.
All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Generation and PCR genotyping of Bco1−/− mice has been described previously (33). Wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in shoebox cages in a temperature- and humidity-controlled facility with ad libitum access to water. Mice were acclimated to powdered AIN-93G diet for 2 to 3 weeks before exposure to experimental diets.
Male wild-type (WT; C57BL/6J background) or Bco1−/− mice (C57BL/6J congenic) consumed chow diet (no. 8640, Harlan Teklad, Indianapolis, IN) from weaning at 3 wk of age until 6–11 wk of age, after which they were maintained on a powdered, semipurified, AIN-93G-based diet (72) for 2–3 wk before euthanasia. Diets were devoid of carotenoids and contained 825 μg retinyl palmitate/kg diet; previous work in our laboratory with this mouse model has demonstrated that this diet is sufficient to maintain normal hepatic and serum vitamin A levels (49). All mice were fasted for 3 h and asphyxiated with CO2; then, blood was collected via cardiac puncture. For histopathology and immunohistochemistry of the testes and prostate, one testicle or one lobe each of the anterior, dorsolateral, and ventral prostate was fixed in 10% neutral-buffered Formalin for 24 h and subsequently transferred to 70% aqueous ethanol until paraffin embedding. All other tissues were snap-frozen in liquid nitrogen and stored at −80°C until analysis. All mice were euthanized between 1300 and 1600 U.S. Central Daylight Time to control for diurnal variation in hormone levels.
Serum hormone analysis.
Serum luteinizing hormone (LH) was measured in neat serum with an ELISA (CEA441Mu, Cloud-Clone, Houston, TX) per the manufacturer’s instructions. Serum steroids were extracted with diethyl ether, and lipids were removed with a biphasic hexane-methanol extraction. Briefly, 150 μl of serum was spiked with ~1,000 cpm of tritiated testosterone (Perkin-Elmer, Waltham, MA) and vortexed for 3 min with 6 ml diethyl ether (Sigma, St. Louis, MO). The aqueous phase was frozen in dry ice-cooled methanol, and the ether extract was removed and evaporated. Hexane and methanol (1.5 ml each; Sigma) were added to each tube and vortexed for 3 min. The hexane and methanol layers were allowed to separate for 30 min, and the bottom methanol layer was removed and dried. Samples were reconstituted in PBS + 1% wt/vol gelatin. Fifteen microliters were removed for scintillation counting and calculation of extraction efficiency, which averaged 75% for both the testosterone and estradiol assays. Serum testosterone was quantified with a RIA kit (TKTT1 Coat-A-Count Total Testosterone, Siemens, Malvern, PA). Serum estradiol was quantified with an RIA (KE2D1 double antibody estradiol; Siemens).
Gene expression.
Total RNA was extracted from testis and prostate tissue using RNEasy mini spin columns (Qiagen, Valencia, CA). cDNA was synthesized using the high-capacity reverse transcription kit (Life Technologies, Grand Island, NY). Quantitaive PCR (qPCR) was carried out with SYBR Green chemistry (Life Technologies) on an ABI 7900 HT qPCR platform (Life Technologies), using predesigned primer assays (Cyp17a1, Hsd17b2, Hsd17b3, Scarb1, Hmgcr, Vim, and Rpl19; SA Biosciences, Valencia, CA) or custom-designed oligonucleotide primers [Integrated DNA Technologies (IDT), Coralville, IA]: Star forward 5′-TTGGGCATACTCAACAACCA-3′, reverse 5′-CCTTGACATTTGGGTTCCAC-3′; Ar forward 5′-TCTTTCAAGGGAGGTTACGC-3′, reverse 5′-AGGACGGGATCTCAAGTGTC-3′; Msmb forward 5′-GTCAATCACCTGCTGTACCAAC-3′, reverse 5′-CTGGGTTCTTCCGATCCAC-3′; Igfbp3 forward 5′-TCCACTCCATGCCAAGATG-3′, reverse 5′-CTGTCTCCCGCTTAGACTCG-3′; Lcat forward 5′-GGATATGTGCGGGATGAGAC-3′, reverse 5′-AGTGGAGCACATGCAGACAG-3′; Soat1 forward 5′-AGCCCAGAGGCTCAATGTTA-3′, reverse 5′-GGCTAGCACAACCACACTGA-3′; Soat2 forward 5′-TGTCACAGAACAGGGCAGAG-3′, reverse 5′-TGACAGTTCCTGTCCCATCA-3′; and Rpl19 forward 5′-AAATCGCCAATGCCAACTC-3′, reverse 5′-ACCCTTCCTCTTCCCTATGC-3′. Oligonucleotide primers were validated for specificity (via verification of a single SYBR dye dissociation curve) and amplification efficiency (via linear slope of Ct vs. serial dilution of template = −3.322 ± 5%).
NanoString nCounter technology was used for prostatic gene expression analysis on a custom-designed 200-gene code set (Supplemental Table S1), which has been described previously (80). RNA was extracted from the dorsolateral prostates of mice as described above and 100 ng total RNA, without reverse transcription to cDNA, was directly used for gene expression analysis (24). Data were subjected to global normalization (global mean fold-change = 1.06; 95% confidence interval: 0.97, 1.14). Transcripts with expression below negative control group means were excluded from further analysis. A heat map was constructed in the nSolver Analysis Software by subjecting Z-scored genes to Ward’s minimum distance linkage within Pearson’s correlation agglomerative hierarchical clustering, based on similarity of gene expression. For identification of gene clusters within the heat map, clusters were defined as 6th-order families or higher, in a structure that contained up to 10th-order families.
Protein expression.
Tissues were lysed in RIPA buffer with the addition of Halt protease inhibitor cocktail, EDTA-free (Pierce Thermo Scientific, Rockford, IL) and were protein quantified using the BCA assay (Pierce Thermo Scientific). After SDS-PAGE and transfer onto PVDF membranes (0.45 μm; Immobilon-P, Millipore), blots were blocked with 5% nonfat dry milk and probed for CYP11A1 (sc-18043, Santa Cruz Biotechnology, Dallas, TX; 1:200 dilution) with GAPDH (no. 5174, Cell Signaling Technology, Danvers, MA; 1:4000 dilution) as the loading control. Androgen receptor expression (no. 06–680, Millipore; 1:500) was measured relative to histone H3 (no. 4499, Cell Signaling Technology; 1:2000) as the loading control. HMG-CoA reductase expression was probed (sc-33827, Santa Cruz Biotechnology; 1:1000) with GAPDH as the loading control. Detection was with donkey anti-goat (sc-2020, Santa Cruz Biotechnology; 1:5000) and goat anti-rabbit (no. 7074, Cell Signaling Technology; 1:2000) HRP-linked secondary antibodies, SuperSignal Molecular Weight Protein Ladder (Pierce Thermo Scientific), and Amersham ECL Prime Western Blotting Reagent (GE Life Sciences, Pittsburgh, PA) on an ImageQuant LAS 4000 imaging station (GE Life Sciences). Relative band densitometry was performed with Fiji software (73) by integration of intensity profile area under the curve.
Testicular cholesterol.
Total testicular lipids were extracted from ~25 mg tissue with the method of Folch et al. (22). Dried lipid extracts were reconstituted in 50 μl of 10% Triton X-100 in isopropanol and sonicated for 30 min. Total, free, and esterified cholesterol were measured with a colorimetric assay per manufacturer’s instructions (ab65359; Abcam, Cambridge, MA).
Histology.
Testes and prostates were fixed in 10% neutral-buffered formalin for 24 h, transferred to 70% ethanol until embedding in paraffin blocks, and cut into 5-μm sections. Cross-sectional testicular sections were stained with periodic acid-Schiff-hematoxylin (PAS-H), and prostatic sections were stained with hematoxylin and eosin (H&E). Staining was done in the Comparative Biosciences Histology Laboratory at the College of Veterinary Medicine, University of Illinois at Urbana-Champaign. Slides were digitized with a NanoZoomer 2.0-HT slide scanner (Hamamatsu, Bridgewater, NJ) equipped with an Olympus Uplansapo 10× objective (Tokyo, Japan). Images were captured with NDP View software (Hamamatsu).
A blinded evaluator with expertise in comparative pathology (Dr. Matthew A. Wallig) assessed testicular and prostatic pathology. Testes were graded on the basis of seminiferous tubule contraction/dilation and the vacuolation, apoptosis, necrosis, and size of Sertoli and Leydig cells (score 1 = <5% affected, 2 = 5–25% affected, 3 = 26–50% affected, 4 = 51–75% affected, and 5 = >75% affected). Prostates were graded on the basis of gland size (score 0 = normal, 1 = slight decrease, 2 = decrease, 3 = marked decrease) and abundance of glandular secretion (score 0 = 0% decrease, 1 = <20% decrease, 2 = 20–40% decrease, 3 = >40% decrease).
Testicular morphology (seminiferous tubule number, total tubule size, tubule lumen area, and tubule epithelial area) was measured in Fiji using the following method: 1) representative frames of H&E-stained testicular sections were captured at ×10 optical magnification; 2) circumference of individual seminiferous tubules, which were no less than 80% within frame, was manually traced and total tubule area (lumen + epithelium; ta) calculated in μm2; 3) lumen circumference of individual seminiferous tubules was manually traced and lumen area (la) calculated in μm2; 5) epithelial cell layer area (ea) of individual seminiferous tubules was calculated as ea = ta - la; 6) seminiferous tubules measured in this fashion were counted; 7) TA, LA, and EA were calculated as the averages of ta, la, and ea, respectively, across all counted seminiferous tubules; and 8) Leydig cells were counted and expressed as cell count/1000 μm2 TA. All calculations were subsampled across 6 or 7 seminiferous tubules/frame and 2 or 3 frames/slide. Averages for an entire slide (i.e., animal/experimental unit) were used for statistical analysis.
Immunofluorescence imaging.
Formalin-fixed, paraffin-embedded prostatic sections were used for immunofluorescence staining. Background Punisher blocking agent (Biocare Medical, Concord, CA) was used to reduce nonspecific binding. Sections were probed for AR or Ki-67 with primary (AR: 1:100, Millipore; Ki-67: 1:600, no. 9129, Cell Signaling Technology) and secondary antibodies (DyLight 488, 1:200, no. 111–487–03–03, Jackson ImmunoResearch Laboratories, West Grove, PA). Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI, 1:1000, no. 32670, Sigma-Aldrich). Negative controls were created for each sample by omission of the primary antibody; nonspecific staining was revealed to be minimal (data not shown). Slides were imaged on a Zeiss LSM 700 confocal microscope at the Core Facilities of the University of Illinois Carl R. Woese Institute for Genomic Biology.
Ki-67 quantitation was performed in Fiji on 20× confocal images using a thresholding-watershed cell counting method (Fiji. Nuclei Watershed Separation. Available from http://imagej.net/Nuclei_Watershed_Separation. Accessed Nov 2, 2015).
Statistical analysis.
Data are presented as means ± SE. With the exception of the NanoString gene expression analysis, all data were analyzed by ANOVA, and results were considered statistically significant at P ≤ 0.05. Data were transformed logarithmically when normality assumptions were violated. Individual group variances were used in place of the pooled variance when homogeneity of variance assumptions was violated. Where appropriate, data were blocked by cohort, assay batch, or euthanasia order. ANOVA analysis was done in SAS v9.3 (SAS Institute, Cary, NC). For NanoString expression analysis, calculations of fold regulation by Bco1−/− genotype (relative to WT), heat map assembly, correlation analysis, and hierarchical clustering were done within the NanoString nSolver Analysis Software (NanoString Technologies, Seattle, WA). Pairwise t-tests were considered significant at α = 0.005. False discovery rate (FDR) at α = 0.005 was estimated to be FDRhat = 0.060 (95% confidence interval: 0.053, 0.068) using the method of Storey (60, 77).
RESULTS
Prostate and seminal vesicle weights are decreased in Bco1−/− mice.
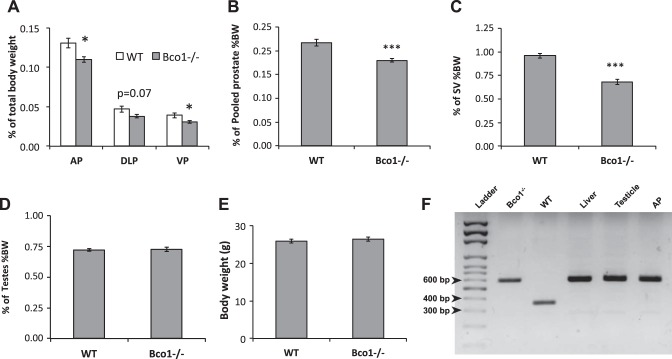
As a percentage of body weight (%BW), weights of the anterior and ventral prostate lobes were significantly decreased (P < 0.05) in Bco1−/− mice, relative to WT (Fig. 1A), while dorsolateral prostate lobe weights trended lower (P = 0.07). Total pooled prostate %BW was significantly decreased by 18% in Bco1−/− mice compared with WT (P < 0.001, Fig. 1B). The seminal vesicles, like the prostate, are dependent upon androgens for growth; similarly, Bco1−/− mice were found to have statistically significant decreases in seminal vesicle %BW, compared with WT (−29%, P < 0.001, Fig. 1C). We observed no effect of Bco1 disruption on body weight or testicular weight (Fig. 1, D and E). Disruption of Bco1 was confirmed in the liver, testes, and prostate of Bco1−/− mice by PCR genotyping (Fig. 1F).
Fig. 1.
Bco1−/− loss reduces seminal vesicular and prostatic weight as a percentage of body weight. A: anterior (AP), dorsolateral (DLP), and ventral prostate (VP) lobe weight percentages of body weight are shown. B: total prostate weight as a percentage of body weight is shown. Seminal vesicles (SV) (C) and testes (D) are also shown as a percentage of total body weight (%BW). E: total body weight. Data are presented as group mean %BW ± SE; n = 15, *P < 0.05, ***P < 0.001. F: PCR genotyping confirms Bco1 disruption in several tissues of male Bco1−/− mice. Lane 1, DNA ladder; lane 2, tail snip from Bco1−/− breeding dam; lane 3, tail snip from wild-type (WT) breeding dam (Jackson Laboratories); lane 4, liver sample from male Bco1−/− study animal; lane 5, testicle sample from Bco1−/− study animal; lane 6, AP sample from Bco1−/− study animal. WT and Bco1−/− band sizes correspond to those reported by Hessel et al. (33). WT: 351 base pairs (bp); Bco1−/−: 588 bp.
Serum testosterone is decreased in Bco1−/− mice.
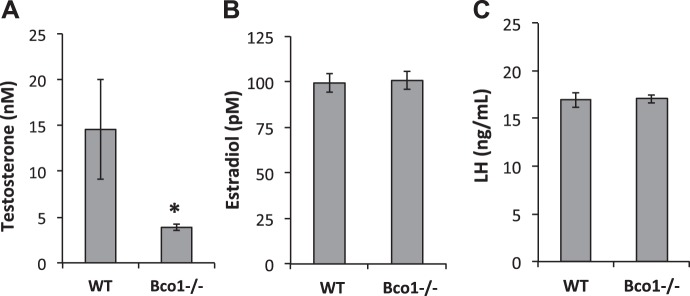
Given the significant reductions in the weights of the prostate and seminal vesicles, we measured serum concentrations of relevant hormones. Serum concentrations of testosterone were significantly lower in Bco1−/− mice than in WT mice (3.9 ± 0.3 nM vs. 14.6 ± 5.4 nM, respectively, P < 0.05; Fig. 2A). Serum concentrations of estradiol were unchanged by genotype (99.3 ± 5.0 pM in WT vs. 100.7 ± 4.8 pM in Bco1−/−, Fig. 2B), as were serum concentrations of LH (16.9 ± 0.79 ng/ml in WT vs. 17.1 ± 0.43 ng/ml in Bco1−/−; Fig. 2C).
Fig. 2.
Serum concentrations of testosterone (A), but not estradiol (B) or luteinizing hormone (LH; C), are reduced by Bco1 disruption. Testosterone and estradiol were measured by RIA in diethyl ether/hexane-methanol extracts of serum, while LH was measured with an ELISA in neat serum. Data are presented as means ± SE; n = 9–13. *P < 0.05.
Bco1 disruption alters testicular morphology and reduces Leydig cell number.
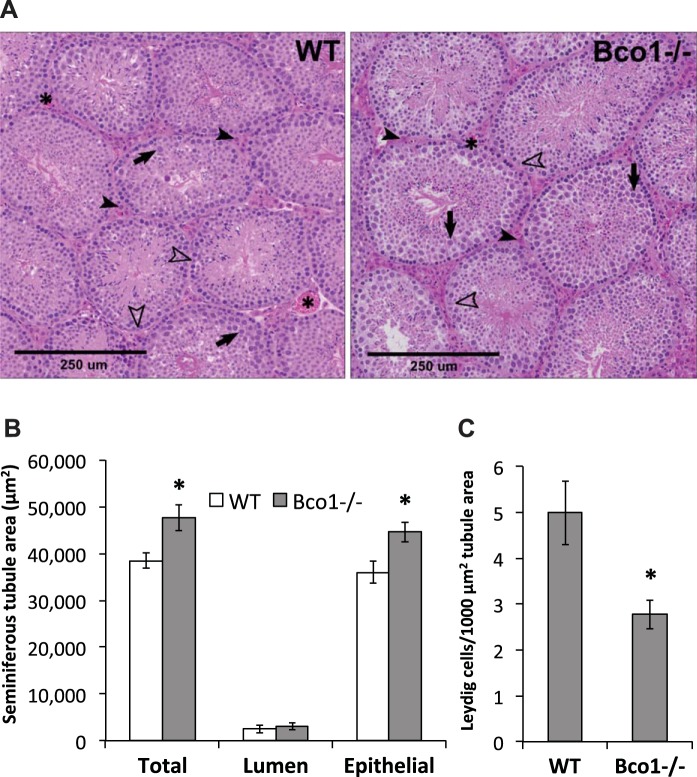
Histopathological assessment of testicular sections from WT and Bco1−/− mice suggested a modest increase in tubule size in Bco1−/− mice compared with WT mice (Fig. 3A). Necrosis, vacuolation of Sertoli or Leydig cells, and alterations in Leydig cell size were all determined to be minimal/normal (data not shown). Morphological quantification corroborated the histopathological finding of tubule enlargement and revealed an increase in total seminiferous tubule area and seminiferous epithelial area—but not lumen area—as a result of Bco1 disruption (Fig. 3B). Counting of Leydig cells (normalized to tubule area) revealed a 44% decrease in Leydig cell number in Bco1−/− mice, compared with WT (Fig. 3C).
Fig. 3.
Histopathological and morphological assessment of testes reveals structural changes with Bco1 loss. A: pathological grading suggested increases in tubule size and moderate reductions in spermatocyte apoptosis in Bco1−/− mice, compared with WT. Representative images are shown; Leydig cells (solid arrowheads) reside in the interstitial space between seminiferous tubules, often alongside blood vessels (asterisks). Cells within the seminiferous tubules include Sertoli cells (open arrowheads) and spermatocytes (solid arrows). Morphological quantification was carried out as described in materials and methods and revealed that Bco1 disruption increased seminiferous tubule total and epithelial areas (B) and decreased Leydig cell number (C); n = 3–5 mice/genotype, *P ≤ 0.05.
Testicular androgen biosynthetic capacity at the mRNA level is reduced in Bco1−/− mice.
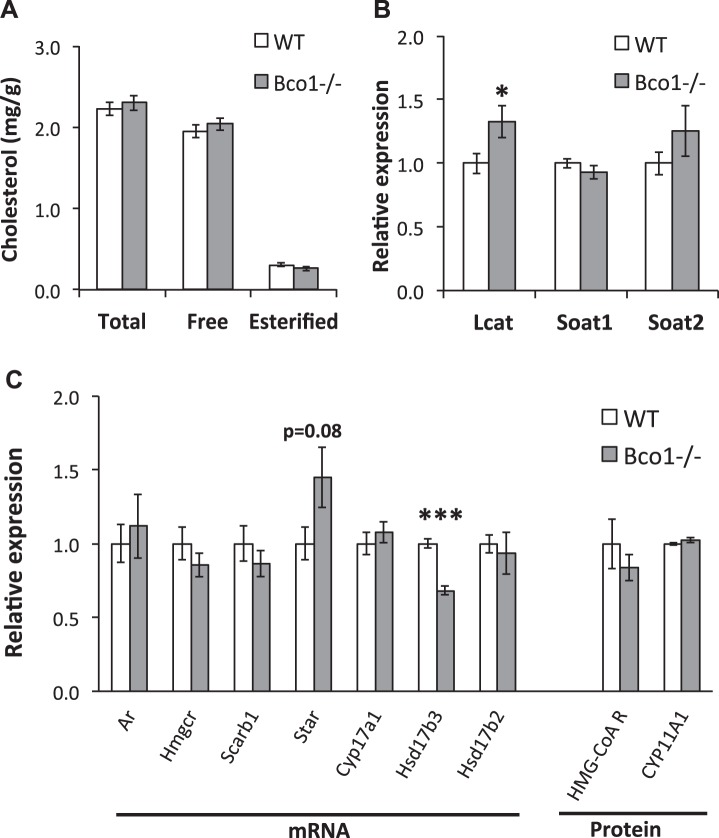
Testicular Leydig cells obtain cholesterol substrate for testosterone synthesis via lipoprotein import or through de novo synthesis. Testicular levels of total, free, and esterified cholesterol were unaltered by Bco1 genotype (Fig. 4A). Two enzymes that catalyze the esterification of cholesterol, Soat1, and Soat2, were unaltered, while a third, Lcat, was significantly increased with Bco1 loss (Fig. 4B). Seven key mediators of testicular cholesterol acquisition (de novo synthesis and selective uptake), steroidogenesis, and androgen metabolism were assayed to determine the effects of Bco1 disruption on testicular steroidogenic potential (Fig. 4C). In agreement with testicular cholesterol concentrations, no differences in testicular expression of genes or proteins controlling de novo cholesterol synthesis (Hmgcr transcript, HMG-CoA reductase protein) or selective cholesterol uptake (Scarb1) were observed. Transcript for steroidogenic regulatory protein (Star), which governs steroidogenesis through intracellular transport of cholesterol to the steroidogenic apparatus, was not significantly modulated by Bco1 loss. Two key mediators of general steroidogenesis were unchanged (CYP11A1 protein, Cyp17a1 transcript), but transcript of Hsd17b3, which encodes an enzyme responsible for the final reduction of androstenedione to testosterone, was significantly decreased by Bco1 disruption (−32%, P < 0.001). Hsd17b2, which encodes a protein that catalyzes the reverse reaction as the enzyme arising from Hsd17b3, was unchanged. Testicular Ar transcript was not altered in Bco1−/− mice, compared with WT.
Fig. 4.
Bco1 loss alters mRNA expression of steroidogenic enzymes in the testes. Testicular cholesterol (A), mRNA expression of cholesterol esterification enzymes (B), and mRNA and protein expression of steroidogenic enzymes (C) were measured. Lcat, Soat1, Soat2, Hmgcr, Scarb1, Star, Cyp17a1, Hsd17b3, and Hsd17b2 mRNA expression was determined by qPCR and HMG-CoA reductase and CYP11A1 protein expression was measured by Western blot analysis. mRNA expression was normalized to Rpl19, and protein expression was normalized to GAPDH. Data are presented as means ± SE; n = 8–18, *P < 0.05, ***P < 0.001.
Prostatic AR signaling and cellular proliferation are reduced in Bco1−/− mice.
Concurrent observations of decreased prostatic weight and reduced serum testosterone concentrations suggest that Bco1 disruption may alter prostatic biology through androgen-dependent pathways. We explored this hypothesis by examining prostatic AR expression and subcellular localization, prostatic proliferation, and the prostatic expression of genes associated with cellular proliferation, cell cycle progression, apoptosis, and prostate carcinogenesis.
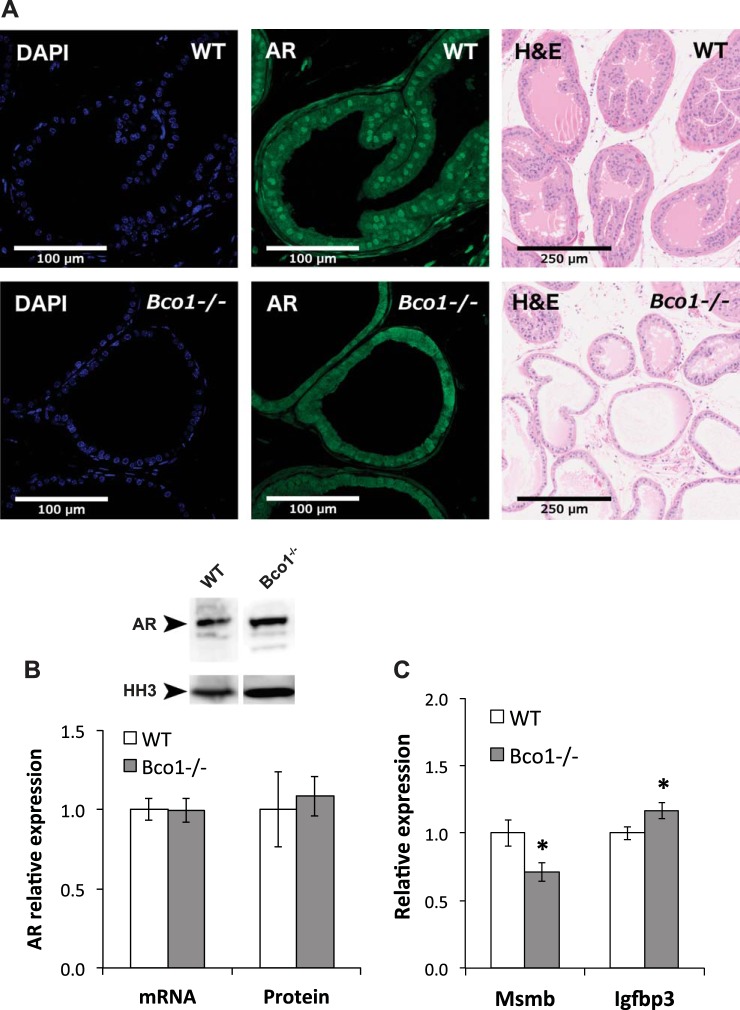
We performed immunofluorescence confocal microscopy on formalin-fixed, paraffin-embedded prostatic sections to evaluate the effect of Bco1 disruption on prostatic AR nuclear localization. Representative images are shown in Fig. 5A, demonstrating a reduction in nuclear AR staining in dorsolateral prostate sections from Bco1−/− mice, compared with WT. Moreover, morphological assessment of H&E-stained dorsolateral prostate sections (Fig. 5A) revealed a concordant effect, with Bco1 loss resulting in smaller gland structures and reduced secretory accumulation, compared with WT (Fig. 5A). These effects were limited to the dorsolateral prostate and were not observed in the ventral or anterior prostate lobes (data not shown). Although Bco1 loss appeared to reduce nuclear localization of AR, we did not detect changes in prostatic AR mRNA by qPCR or protein by Western blot analysis (Fig. 5B). Demonstrating a functional outcome of the observed reduction in AR nuclear localization, prostatic mRNA expression of the androgen-induced AR target Msmb was significantly reduced in Bco1−/− mice, while the androgen-repressed gene Igfbp3 was significantly induced (Fig. 5C).
Fig. 5.
Bco1 disruption altered prostatic morphology, reduced prostatic AR localization, and altered prostatic androgen signaling, but did not impact androgen receptor (AR) expression. A: Bco1 disruption reduced nuclear AR localization within the dorsolateral prostate (second column) and altered dorsolateral prostate morphology and glandular secretion (third column). Representative images of n = 3 or 4 mice per genotype are shown for AR staining; H&E images are representative of n = 9 or 10 mice/genotype. B: AR mRNA and protein expression are unchanged in Bco1−/− mice. C: prostatic androgen signaling is altered in Bco1−/− mice, as demonstrated by a reduction in mRNA expression of the androgen-induced gene Msmb and an induction of the androgen-repressed gene Igfbp3. Data are presented as means ± SE; n = 11–25 for quantitative PCR and n = 5 for Western blotting (WB). Ar, Msmb, and Igfbp3 mRNA expression were normalized to Rpl19 and histone H3 (HH3) was used as a loading control for Western blot analyses. *P < 0.05.
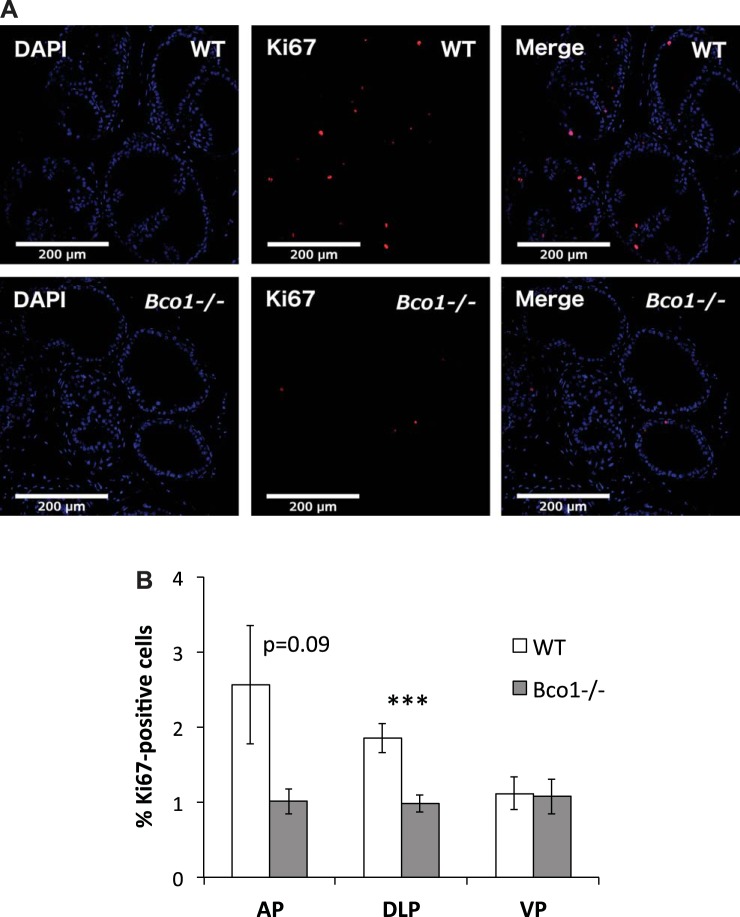
We next assessed prostatic proliferation through Ki-67 immunofluorescence confocal microscopy (Fig. 6A). Ki-67 labeling (as a percentage of total nuclei) was reduced by nearly half in the dorsolateral prostate of Bco1−/− mice (1.85% ± 0.19% in WT vs. 0.98% ± 0.11% in Bco1−/−, P < 0.001) and trended lower in the anterior prostate (2.56% ± 0.78% in WT vs. 1.01% ± 0.17% in Bco1−/−, P = 0.09) (Fig. 6B).
Fig. 6.
Bco1−/− disruption reduces immunofluorescent labeling of Ki-67 protein in the dorsolateral prostate. A: representative images of the dorsolateral prostate are shown. B: quantification through cell counting in the anterior (AP), dorsolateral (DLP), and ventral prostate (VP) is shown. n = 5 or 6; ***P < 0.001.
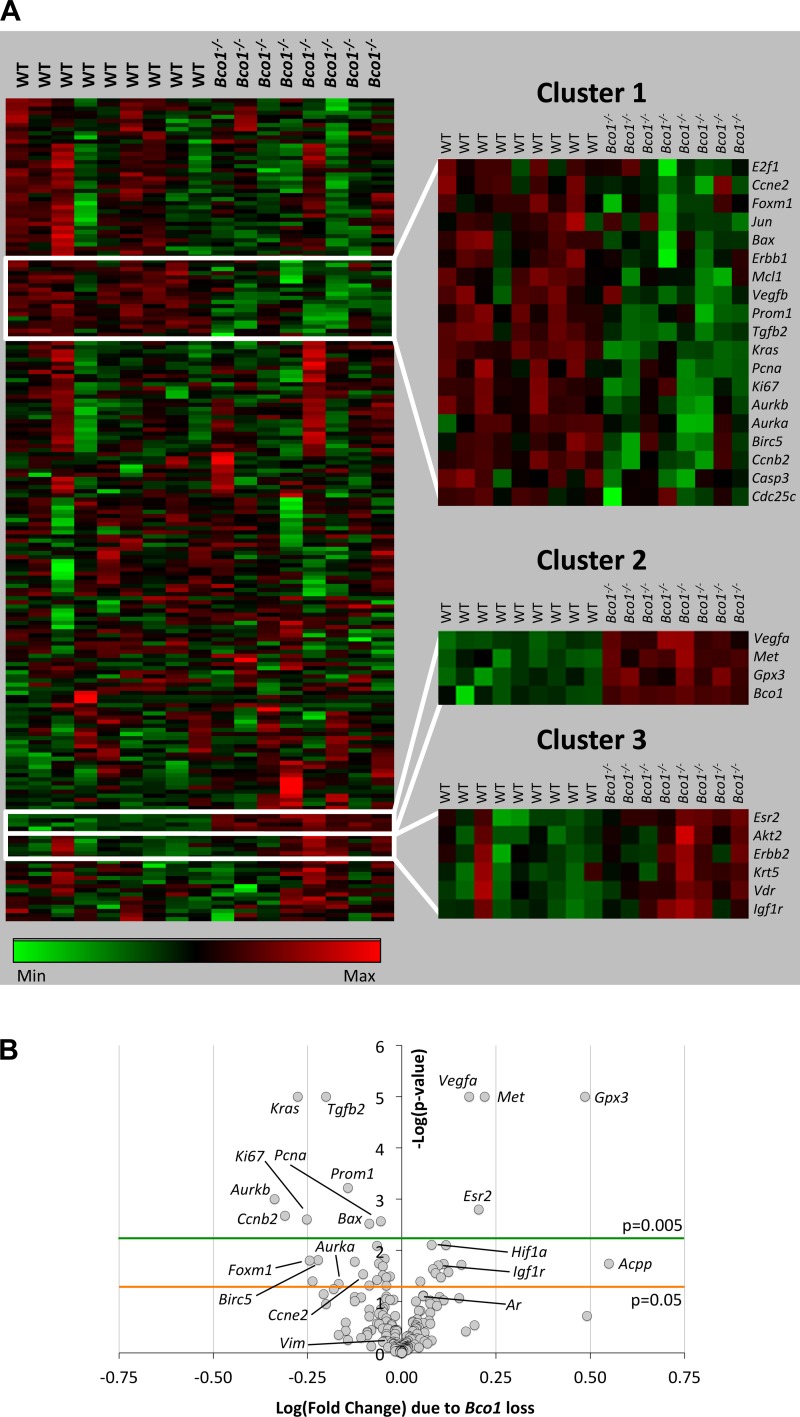
Because of our observations of reduced AR nuclear localization and cellular proliferation in the dorsolateral prostates of Bco1−/− mice, we examined gene expression using our previously described custom NanoString array (80). This 200-gene array includes genes involved in prostatic carcinogenesis, proliferation, cell cycle progression, and apoptosis (Fig. 7A). Of the 200 genes, 40 genes were regulated by Bco1 loss at P < 0.05 and 56 at P < 0.10 (Supplemental Table S1). Bco1 disruption significantly (P < 0.005) modulated 13 genes of the 200-gene code set (Fig. 7B and Table 1). Among the 13 genes significantly altered (P < 0.005) by Bco1 loss were genes involved in proliferation (Ki67, −44%; Pcna, −12%; Prom1, −28%), cell cycle progression (Aurkb, −54%; Ccnb2, −51%), apoptosis (Bax, −18%), and stromal-epithelial growth factor regulation (Tgfb2, −37%; Met, +66%). When data were assembled into a heat map using hierarchical clustering of gene expression, the 13 genes significantly modulated at P < 0.005 were distributed among three clusters, which collectively contained a total of 29 genes (Fig. 7A; dendrogram not shown). Correlation analysis of each of the three cluster nodes revealed that the expression of genes within each was highly correlated (Pearson’s r2 ≥ 0.93), suggesting functional similarity or common regulation in Bco1−/− mice. Indeed, while 16 of the 29 genes within these three clusters were nonsignificantly regulated at P > 0.005, many of these 16 were also related to apoptosis (Birc5, −40%; P = 0.015), cell cycle progression (Aurka, −32%; P = 0.045; Ccne2, −21%; P = 0.029; Foxm1, −43%; P = 0.029; E2f1, −38%; P = 0.071), and stromal-epithelial growth factor regulation (Igf1r, +25%; P = 0.020; Erbb1, −14%; P = 0.037) (Table 1). All of the genes in the 200-gene array that were significantly modulated by Bco1 loss were contained within the three clusters highlighted in Fig. 7A, and 25 of the 29 genes within the three clusters were differentially expressed in WT and Bco1−/− mice with P values below 0.05.
Fig. 7.
Prostatic gene expression is altered in Bco1−/− mice. NanoString quantitative gene expression analysis for 200 proliferation-, apoptosis-, androgen-, and cancer-related genes WT or Bco1−/− mice (n = 8 or 9) displayed in a heat map (A) and volcano plot (B). Z-scored genes (rows) were subjected to Ward’s minimum distance linkage within Pearson’s correlation hierarchical clustering (A; dendrogram not shown). B: absolute log (fold-change) values of 0.25 and 0.50 approximately equal absolute fold-regulation values of 1.8 and 3.2, respectively. A: clusters of correlated genes with differential expression between the WT and Bco1−/− genotypes are highlighted. Complete data for all 200 genes are displayed in Supplemental Table S1.
Table 1.
Genes differentially regulated in prostates of Bco1−/− mice
| Symbol | Gene Name | Gene Ontology Process | Fold Change | P Value |
|---|---|---|---|---|
| Cluster 1 | ||||
| E2f1 | E2F transcription factor 1 | Cell cycle regulation | 0.62 | 0.0708 |
| Ccne2 | Cyclin E2 | Cell cycle regulation | 0.79 | 0.0291 |
| Foxm1 | Forkhead box M1 | Transcription factor | 0.57 | 0.0157 |
| Jun | Jun proto-oncogene (AP-1) | Cell cycle regulation | 0.82 | 0.0484 |
| Bax | BCL2-associated X protein | Apoptosis | 0.82 | 0.0030 |
| Erbb1 | Epidermal growth factor receptor | Proliferation; apoptosis | 0.86 | 0.0376 |
| Mcl1 | Myeloid cell leukemia sequence 1 | Apoptosis | 0.90 | 0.0146 |
| Vegfb | Vascular endothelial growth factor B | Growth factor | 0.87 | 0.0179 |
| Prom1 | Prominin 1 (Cd133) | Proliferation; stem cell marker | 0.72 | 0.0006 |
| Tgfb2 | Transforming growth factor, β2 | Proliferation; apoptosis | 0.63 | <0.0001 |
| Kras | Kirsten rat sarcoma viral oncogene | Intracellular signaling | 0.53 | <0.0001 |
| Pcna | Proliferating cell nuclear antigen | Proliferation | 0.88 | 0.0027 |
| Ki67 | Marker of proliferation Ki-67 | Proliferation | 0.56 | 0.0025 |
| Aurkb | Aurora kinase B | Cell cycle regulation | 0.46 | 0.0005 |
| Aurka | Aurora kinase A | Cell cycle regulation | 0.68 | 0.0449 |
| Birc5 | Survivin | Apoptosis | 0.60 | 0.0154 |
| Ccnb2 | Cyclin B2 | Cell cycle regulation | 0.49 | 0.0021 |
| Casp3 | Caspase 3 | Apoptosis | 0.93 | 0.0320 |
| Cdc25c | Cell division cycle 25C | Cell cycle regulation | 0.63 | 0.1108 |
| Cluster 2 | ||||
| Vegfa | Vascular endothelial growth factor A | Angiogenesis | 1.51 | <0.0001 |
| Met | HGF/SF receptor | Growth factor receptor | 1.66 | <0.0001 |
| Gpx3 | Glutathione peroxidase 3 | Catalytic activity | 3.06 | <0.0001 |
| Bco1 | β-Carotene oxygenase 1 | Catalytic activity | 8.21 | 0.0001 |
| Cluster 3 | ||||
| Esr2 | Estrogen receptor 2 (β) | Nuclear hormone receptor | 1.60 | 0.0016 |
| Akt2 | Protein kinase Bβ | Cell cycle regulation; apoptosis | 1.23 | 0.0276 |
| Erbb2 | Her2/Neu receptor | Proliferation; apoptosis | 1.12 | 0.1489 |
| Krt5 | Keratin 5 | Cytoskeleton | 1.29 | 0.0943 |
| Vdr | Vitamin D receptor | Nuclear hormone receptor | 1.21 | 0.0232 |
| Igf1r | Insulin-like grwoth factor 1 receptor | Growth factor receptor | 1.25 | 0.0196 |
Bco1 disruption alters the prostatic expression of genes involved in cell cycle progression, proliferation, apoptosis, and prostate carcinogenesis. Clusters consist of significantly modulated (P < 0.005) genes and other genes that were correlated (R2 ≥ 0.93) with their expression. These gene clusters are also highlighted in Fig. 7A. Gene names and symbols were obtained from the Online Mendelian Inheritance in Man (OMIM) database. Cellular/molecular process classification was done according to gene ontology annotations in the PANTHER Classification System. P values ≤ 0.005 are in bold.
DISCUSSION
This work sought to explore the effects of disrupted Bco1 function on androgen status and prostatic biology in mice. On a broader scale, it was undertaken to better understand BCO1 function and the potential physiological impacts of genetic variability in the human BCO1 gene. We observed a reduction in serum testosterone concentration due to Bco1 disruption, which may have been driven by a decrease in testicular Leydig cell populations. Concomitantly, compared with WT animals, Bco1−/− mice exhibited altered prostatic morphology and reductions in prostatic markers of androgen signaling, proliferation, and cell cycle progression. Together, these data support a hypothesis that Bco1−/− mice experience reduced testicular testosterone synthesis and lowered concentrations of circulating testosterone, resulting in depressed prostatic androgen signaling, proliferation, and organ weight.
Both the prostate and seminal vesicles, as male sex accessory glands, are dependent upon androgens for growth and homeostasis (34, 35, 78). We found that seminal vesicular and prostatic weight was reduced in Bco1−/− mice, with approximately a 20% reduction in each of the three microdissected prostate lobes. Other organs, such as the testes (Fig. 1D), brain, kidneys, lungs, and adrenals (data not shown) were not impacted by Bco1 loss. In conjunction with a reduction in circulating testosterone in Bco1−/− mice, these data suggest that Bco1 disruption may specifically impact androgen-sensitive tissues.
In the testes, testosterone is produced by Leydig cells (68), which express all of the enzymes necessary for androgen synthesis (64, 65). Previous reports demonstrate that reductions in Leydig cell number are associated with reduced testosterone production (37, 51, 52). Thus, we examined histological sections of the testes from WT and Bco1−/− mice to assess morphology and Leydig cell populations. Leydig cell number, normalized to seminiferous tubule area, was lower in Bco1−/− testes than in WT testes. Leydig cell counts were normalized to tubule area, rather than to total area or tubule count, because variation in tubule diameter necessarily alters tubule packing density, and therefore, the volume of interstitial space where Leydig cells reside. Reduced populations of Leydig cells in Bco1−/− mice may have contributed to the diminished levels of circulating testosterone observed with Bco1 loss. However, as no differences in Leydig cell apoptosis or necrosis were identified via histopathology, it remains unclear how this defect manifests in the testes of the Bco1−/− mice. We also found that disruption of Bco1 increased total seminiferous tubule area, driven by a modest increase in seminiferous epithelial area. Qualitatively, this appeared to be driven by an increase in spermatocyte number. It is well established that spermatogenesis requires androgens, particularly to progress past the spermatocyte stage to meiosis (58). Sertoli cell-specific AR knockout yields an accumulation of premeiotic spermatocytes (79), but, in contrast to our results, also reduces testicular weight and seminiferous tubule diameter. It is not clear why we observe some aspects of a testicular phenotype consistent with testosterone deficiency, but not others. The current study was not designed to assess spermatogenesis or fertility end points, but given our morphological observations, future investigation seems warranted to more clearly determine the testicular impact of Bco1 disruption.
In men, it is estimated that up to half of the androgen available to the prostate is derived from local conversion of adrenal steroid precursors, whereas the remainder is supplied directly as testicle-derived testosterone (40). Rodents, however, produce little adrenal androgen precursor steroids, and the prostate in these species relies on testicular testosterone for androgenic activity (15, 84). Androgen synthesis is a multistep process that is dependent upon several key enzymes for acquisition of cholesterol substrate, followed by conversion to active androgens (64). Expression of mediators of testicular cholesterol synthesis (3-hydroxy-3-methylglutaryl-CoA reductase mRNA and protein) and uptake (scavenger receptor class B member 1 mRNA) was unchanged by Bco1 disruption, in agreement with concentrations of testicular total cholesterol. Esterification of free cholesterol to cholesteryl esters reduces the free cholesterol pool available for active steroidogenesis (6). Three genes play a role in testicular cholesterol esterification: Soat1 and Soat2, whose protein products esterify intracellular cholesterol before transport to the ER for storage in lipid droplets (3, 61), and Lcat, which participates in reverse cholesterol transport and thus export of cholesterol to the liver via HDL (83). While testicular Soat1 and Soat2 were not altered by Bco1 status, Lcat expression was elevated 32% in Bco1−/− mice. However, no differences in free or esterified testicular cholesterol were found, suggesting that the elevation of the low-abundance Lcat transcript (average Ct = 28.3) did not modulate the intratesticular esterified cholesterol pool. Our data do not support a hypothesis that Bco1 disruption reduces serum testosterone through direct modulation of testicular cholesterol metabolism.
LH is released from the pituitary gland as a critical regulator of testicular steroidogenesis (6, 65), controlling the expression and activity of several testicular enzymes, including the transcriptional regulation of Star (56), the rate-limiting step of steroidogenesis (17, 76). We observed no impact of Bco1 disruption on serum LH concentrations or testicular Star expression. It appears that alterations in serum testosterone in Bco1−/− mice may be independent of pituitary gonadotropin stimulation. Additionally, no differences in expression were detected for mediators of two crucial steps of steroidogenesis (CYP11A1 protein and Cyp17a1 transcript), suggesting that decreased serum testosterone levels in Bco1−/− mice were not a result of the regulation of rate-limiting steps of steroidogenesis.
The final step of testosterone synthesis involves the reduction of the inactive androgen, androstenedione, to testosterone via 17β-hydroxysteroid dehydrogenase (17β-HSD), isoform 3 (gene name Hsd17b3) (7, 64). Germline mutations in the human HSD17B3 gene result in deficient testosterone production and subsequent male pseudohermaphroditism (25). Relative to WT, transcript levels of Hsd17b3 were decreased 32% in Bco1−/− mice. 17β-HSD2 (Hsd17b2) catalyzes the reverse, oxidative reaction, converting testosterone back to inactive androstenedione (55); no differences in testicular Hsd17b2 expression between WT and Bco1−/− mice were detected. As testicular Hsd17b3 is expressed exclusively in Leydig cells in adult mice (57), reduced Hsd17b3 expression may be a result of lower Leydig cell counts in Bco1−/− mice, as reported above. However, the proximate mechanism(s) through which Bco1 disruption impact(s) testicular Leydig cell number and/or expression of Hsd17b3 deserve(s) future study.
Androgens mediate their actions at target tissues through high-affinity binding to the AR and subsequent AR-dependent transcriptional regulation of genes containing androgen response elements (AREs). Unliganded AR exists primarily as a monomer in the cytoplasm (27), but upon ligand binding, translocates to the nucleus and recruits transcriptional machinery to AREs to initiate a transcriptional program collectively referred to as “AR signaling” (9, 74). Genes activated in this AR signaling program maintain epithelial differentiation, stimulate release of stromal derived growth factors, and inhibit epithelial apoptosis (10, 11, 38). Translocation of ligand-bound AR from the cytoplasm to t he nucleus during AR signaling is thus a necessary step in androgen-dependent maintenance of the prostate and can serve as an indicator of active androgen signaling (27, 86). WT mice displayed both nuclear and cytoplasmic AR staining within the luminal epithelial cells of the dorsolateral prostate, but many Bco1−/− prostates lacked the punctate nuclear localization characteristic of an androgen-stimulated state. This was limited to the dorsolateral prostate, as AR localization in anterior and ventral prostate sections did not differ between WT and Bco1−/− mice.
We have previously shown that removal of androgenic stimulation by castration reduces prostatic Ki-67 immunolabeling in mice (80). Here, we found that Ki-67 labeling was reduced in the dorsolateral prostates of mice with globally disrupted Bco1. Prostatic AR mRNA and protein expression remained unchanged, indicating that any androgen-regulated effects in the prostates of Bco1−/− mice were likely due to diminished AR activation by circulating androgen and not reduced prostatic expression of the receptor. To our knowledge, screenings of tissue- or cell-specific BCO1 protein expression have not been completed in the mouse. However, although studies in mice have not directly compared testicular and prostatic Bco1 mRNA expression, Bco1 expression in the testes has been shown to be similar or greater to that in the liver and kidney, organs where it is known to be abundantly expressed (62, 71). In addition, immunohistochemical studies in human tissues suggest that BCO1 protein is expressed to a stronger degree in the testes than the prostate; moderate staining was observed in both the Sertoli and Leydig cells of the testis, while only weak staining was seen in prostatic epithelia and the stroma was negative for BCO1 (47). Therefore, the dysregulation of androgen signaling observed in the prostates of Bco1−/− mice is most likely secondary to perturbations in testosterone production caused by testicular Bco1 disruption.
Expression analysis of AR-regulated genes by qPCR supported the conclusion that prostatic androgen signaling is disrupted in Bco1−/− mice. Msmb is an androgen-induced gene and has been shown to be a primary target of AR (39, 54, 82), while Igfbp3, a negative regulator of insulin-like growth factor 1 (IGF1) signaling and primary target of AR, is repressed by androgen (5, 82). As would be expected during conditions of reduced AR signaling, prostatic Msmb expression was significantly reduced by Bco1 loss, while Igfbp3 expression was upregulated.
Prostatic mRNA expression of inducers or markers of cell cycle progression and proliferation [Pcna, Ki67, Aurkb, Ccnb2 (2, 12, 16, 26, 70)] were significantly reduced by Bco1 loss (P < 0.005). Several other related genes [Aurka, Ccne2, Foxm1, Birc5 (1, 36, 69, 87)] were inhibited at 0.005 < P < 0.05 and correlated with significantly modulated genes, clustering near them within the heat map. Some genes, such as Acpp, were not included in any of the three highlighted clusters, despite being relatively highly modulated by Bco1 disruption and having a relatively low P value. We could have chosen to selectively focus on data points such as Acpp. In contrast, using our data-driven hierarchical clustering approach, 25 of 29 genes in the three clusters had P values less than P = 0.05, which, at 86% of the group, is 17-fold higher than would be expected by chance with α = 0.05. In addition, genes within a cluster often shared common ontologies (Table 1). This suggests that our approach has identified the 29 genes within this array that may be the most relevant to understanding the effects of Bco1 disruption on the prostate, as opposed to an approach focused only on highly modulated genes. Indeed, using the same NanoString code set as in this experiment, our group has previously found that castration significantly reduced prostatic mRNA expression of many of the same cell growth and proliferation markers that were inhibited by Bco1−/− loss in the current study [Ki67, Aurkb, Ccnb2, Ccne2, Foxm1, and Birc5 (80)]. These data support the notion that androgen deprivation, whether by castration or loss of Bco1−/−, modulates proliferation in the mouse prostate.
Interestingly, Bco1 mRNA expression was increased 8.21-fold in the prostates of Bco1−/− mice, compared with WT mice. Personal communication with a senior author (J. von Lintig) of the original report describing this model (33) revealed that others have also found the mutant Bco1 mRNA to be expressed in Bco1−/− mice. When Hessel et al. (33) created this genetically engineered model, disruption of the Bco1 gene was achieved through homozygous replacement of exons 2 and 3 (~8.8 kb) with IRES-lacZ and a neomycinR cassette. This was confirmed by PCR genotyping by the original investigators, and the Bco1−/− genotypes of mice used in this study were confirmed by PCR (Fig. 1F). Mice of this strain do not express BCO1 protein, as confirmed by Western blot using antiserum to full-length recombinant mouse BCO1 (33, 63). Additionally, these mice accumulate intact β-carotene when fed β-carotene-containing diets (33, 49), consistent with a lack of BCO1 cleavage function. Moreover, sequencing of the full-length Bco1−/− cDNA reveals not only the deletion of exons 2 and 3 (as expected), but also the fusion of exons 1 and 4. This fusion results in a premature stop codon within the mutant ORF, producing a cDNA that encodes a peptide of only 38 residues, rather than the 566-residue protein present in wild-type mice (J. von Lintig, personal communication). Of importance to our findings, this stop codon occurs before the sequence in exons 9 and 10 targeted by our NanoString primer (Supplemental Table S1; see footnote †). Therefore, the mRNA transcript detected by our assay would not be translated in Bco1−/− mice. Combined with the previously published work summarized above, these data clearly demonstrate that Bco1−/− mice do not produce a functional BCO1 protein. The Bco1 mRNA we have observed in the prostates of Bco1−/− mice encodes a truncated and nonprotein-coding transcript, while its increased expression may represent a futile transcriptional feedback response to the absence of functional Bco1 protein. While Bco1 regulation by transcription factors such as PPARγ and ISX has been well characterized (14, 28, 53, 85), these findings raise the possibility of transcriptional autoregulation of Bco1. Clearly, further work is needed to address this hypothesis.
Together, our data suggest that lowered prostatic AR activation, mediated by reduced serum testosterone due to testicular Bco1 disruption, resulted in decreased AR signaling within the prostate, reduced cellular proliferation, and a decreased growth of the prostate. Moreover, the prostatic gene and protein expression patterns observed in Bco1−/− mice demonstrated a similar phenotype to that previously observed after castration in mice (80).
Perspectives and Significance
Prior studies in mice have shown that adiposity (4), diet-induced obesity (33), hepatic steatosis (33), adipocyte gene expression (4, 33), cholesterol esterification (19), triglyceride and phospholipid metabolism (19), serum insulin, leptin, and cholesterol (23), and cardiac lipid metabolism (43) are all impacted by Bco1 disruption. In conjunction with this previous work, our findings continue to build a convincing argument for the existence of functions of BCO1 that are independent of carotenoid cleavage. Eleven studies (13, 18, 20, 21, 31, 32, 44, 45, 53, 66, 81) have investigated the impact of single nucleotide polymorphisms (SNPs) in the human BCO1 gene. These SNPs are common (most with a minor allele frequency between 0.25 and 0.49) and have been shown to impact carotenoid metabolism and distribution. However, only two of the studies have investigated primary end points other than carotenoid metabolism and distribution; Clifford et al. (18) found a BCO1 SNP to influence serum HDL levels, while Hendrickson et al. (32) reported no associations between BCO1 SNPs and breast cancer risk. Greater understanding of the consequences of Bco1 disruption in the current animal model may not only uncover new aspects of carotenoid metabolism, but also inform the scientific community of potential physiological repercussions of natural genomic variation in the human BCO1 gene. This report adds novel data to the growing body of evidence demonstrating that, although cleavage of dietary carotenoids appears to be a major function of Bco1, its pleiotropic roles in mammalian physiology may extend far beyond carotenoid metabolism.
GRANTS
This study was funded by National Institutes of Health Grant R01-CA-125384.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W.S., N.A.F., E.C.B., S.K.C., and J.W.E. conceived and designed the research; J.W.S., N.A.F., J.M.T.-A., and M.A.W. performed experiments; J.W.S. analyzed data; J.W.S., M.A.W., S.K.C., and J.W.E. interpreted results of experiments; J.W.S. prepared figures; J.W.S. drafted manuscript; J.W.S., N.A.F., J.M.T.-A., N.E.M., E.C.B., M.A.W., S.K.C., and J.W.E. edited and revised manuscript; J.W.S., N.A.F., J.M.T.-A., N.E.M., E.C.B., M.A.W., S.K.C., and J.W.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Johannes von Lintig for sharing data regarding the generation of the Bco1−/− model, Dr. Janice Bahr and Dr. Annie Newell-Fugate for training on radioimmunoassays, Dr. Rex Hess for advice on analysis of testicular morphology, Karen Doty for immunohistochemical and immunofluorescent staining, and Taylor Durkin and Connor Buchweitz for their assistance in the laboratory.
Present addresses: J. W. Smith, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205. N. A. Ford, Nutrition Research, Hass Avocado Board, Irvine, CA 92602. N. E. Moran, Baylor College of Medicine/USDA Children’s Nutrition Research Center, Houston, TX 77030.
REFERENCES
- 1.Addepalli MK, Ray KB, Kumar B, Ramnath RL, Chile S, Rao H. RNAi-mediated knockdown of AURKB and EGFR shows enhanced therapeutic efficacy in prostate tumor regression. Gene Ther 17: 352–359, 2010. doi: 10.1038/gt.2009.155. [DOI] [PubMed] [Google Scholar]
- 2.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst 91: 1869–1876, 1999. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 3.Akpovi CD, Murphy BD, Erickson RP, Pelletier R-M. Dysregulation of testicular cholesterol metabolism following spontaneous mutation of the niemann-pick c1 gene in mice. Biol Reprod 91: 42, 2014. doi: 10.1095/biolreprod.114.119412. [DOI] [PubMed] [Google Scholar]
- 4.Amengual J, Gouranton E, van Helden YGJ, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, von Lintig J. β-Carotene reduces body adiposity of mice via BCMO1. PLoS One 6: e20644, 2011. doi: 10.1371/journal.pone.0020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology 147: 257–271, 2006. doi: 10.1210/en.2005-0942. [DOI] [PubMed] [Google Scholar]
- 6.Azhar S, Reaven E. Regulation of Leydig cell cholesterol metabolism. In: Contemporary Endocrinology: The Leydig Cell in Health and Disease, edited by Payne AH and Hardy MP. New York: Springer, 2007, p. 135–148. doi: 10.1007/978-1-59745-453-7_8. [DOI] [Google Scholar]
- 7.Baker PJ, Sha JH, O’Shaughnessy PJ. Localisation and regulation of 17β-hydroxysteroid dehydrogenase type 3 mRNA during development in the mouse testis [Online]. Mol Cell Endocrinol 133: 127–133, 1997. doi: 10.1016/S0303-7207(97)00159-7. [DOI] [PubMed] [Google Scholar]
- 8.Barua AB, Olson JA. β-Carotene is converted primarily to retinoids in rats in vivo. J Nutr 130: 1996–2001, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Tindall DJ. Androgen action in prostate cancer. Horm Cancer 1: 223–228, 2010. doi: 10.1007/s12672-010-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol 288: 30–37, 2008. doi: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21: 2005–2017, 2007. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bono AV, Montironi R, Pannellini T, Sasso F, Mirone V, Musiani P, Iezzi M. Effects of castration on the development of prostate adenocarcinoma from its precursor HGPIN and on the occurrence of androgen-independent, poorly differentiated carcinoma in TRAMP mice. Prostate Cancer Prostatic Dis 11: 377–383, 2008. doi: 10.1038/pcan.2008.13. [DOI] [PubMed] [Google Scholar]
- 13.Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand J-M, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med 43: 47–59, 2011. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- 14.Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM. Identification of β-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J 17: 1304–1306, 2003. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- 15.Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry 38: 1598–1606, 1999. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- 16.Chieffi P, Cozzolino L, Kisslinger A, Libertini S, Staibano S, Mansueto G, De Rosa G, Villacci A, Vitale M, Linardopoulos S, Portella G, Tramontano D. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate 66: 326–333, 2006. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 17.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269: 28314–28322, 1994. [PubMed] [Google Scholar]
- 18.Clifford AJ, Rincon G, Owens JE, Medrano JF, Moshfegh AJ, Baer DJ, Novotny JA. Single nucleotide polymorphisms in CETP, SLC46A1, SLC19A1, CD36, BCMO1, APOA5, and ABCA1 are significant predictors of plasma HDL in healthy adults. Lipids Health Dis 12: 66, 2013. doi: 10.1186/1476-511X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon JL, Kim Y-K, Brinker A, Quadro L. Loss of β-carotene 15,15′-oxygenase in developing mouse tissues alters esterification of retinol, cholesterol and diacylglycerols. Biochim Biophys Acta 1841: 34–43, 2014. doi: 10.1016/j.bbalip.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feigl B, Morris CP, Voisey J, Kwan A, Zele AJ. The relationship between BCMO1 gene variants and macular pigment optical density in persons with and without age-related macular degeneration. PLoS One 9: e89069, 2014. doi: 10.1371/journal.pone.0089069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi A-M, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 84: 123–133, 2009. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 23.Ford NA, Elsen AC, Erdman JW Jr. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res 33: 733–742, 2013. doi: 10.1016/j.nutres.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26: 317–325, 2008. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 25.Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 β-hydroxysteroid dehydrogenase 3. Nat Genet 7: 34–39, 1994. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 26.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31: 13–20, 1983. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 27.Gerdes MJ, Dang TD, Larsen M, Rowley DR. Transforming growth factor-beta1 induces nuclear to cytoplasmic distribution of androgen receptor and inhibits androgen response in prostate smooth muscle cells. Endocrinology 139: 3569–3577, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Gong X, Tsai S-W, Yan B, Rubin LP. Cooperation between MEF2 and PPARγ in human intestinal β,β-carotene 15,15′-monooxygenase gene expression. BMC Mol Biol 7: 7, 2006. doi: 10.1186/1471-2199-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman DS, Huang HS. Biosynthesis of vitamin A with rat intestinal enzymes. Science 149: 879–880, 1965. doi: 10.1126/science.149.3686.879. [DOI] [PubMed] [Google Scholar]
- 30.van Helden YGJ, Godschalk RWL, Swarts HJM, Hollman PCH, van Schooten FJ, Keijer J. β-Carotene affects gene expression in lungs of male and female Bcmo1 (−/−) mice in opposite directions. Cell Mol Life Sci 68: 489–504, 2011. doi: 10.1007/s00018-010-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickson SJ, Hazra A, Chen C, Eliassen AH, Kraft P, Rosner BA, Willett WC. β-Carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am J Clin Nutr 96: 1379–1389, 2012. doi: 10.3945/ajcn.112.034934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrickson SJ, Lindström S, Eliassen AH, Rosner BA, Chen C, Barrdahl M, Brinton L, Buring J, Canzian F, Chanock S, Clavel-Chapelon F, Figueroa JD, Gapstur SM, Garcia-Closas M, Gaudet MM, Haiman CA, Hazra A, Henderson B, Hoover R, Hüsing A, Johansson M, Kaaks R, Khaw KT, Kolonel LN, Le Marchand L, Lissowska J, Lund E, McCullough ML, Peplonska B, Riboli E, Sacerdote C, Sánchez MJ, Tjønneland A, Trichopoulos D, van Gils CH, Yeager M, Kraft P, Hunter DJ, Ziegler RG, Willett WC. Plasma carotenoid- and retinol-weighted multi-SNP scores and risk of breast cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev 22: 927–936, 2013. doi: 10.1158/1055-9965.EPI-13-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 282: 33553–33561, 2007. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 34.Huggins C, Clark PJ. Quantitative studies of prostatic sectretion II: The effect of castration and of estrogen injection on the normal and on the hyperplastic prostate glands of dogs. J Exp Med 72: 747–762, 1940. doi: 10.1084/jem.72.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg 115: 1192–1200, 1942. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, Lyubimov A, Costa RH. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res 66: 1712–1720, 2006. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J-H, Park S-J, Kim T-S, Kim J-M, Lee D-S. Testosterone production by a Leydig tumor cell line is suppressed by hyperthermia-induced endoplasmic reticulum stress in mice. Life Sci 146: 184–191, 2016. doi: 10.1016/j.lfs.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Kurita T, Wang YZ, Donjacour AA, Zhao C, Lydon JP, O’Malley BW, Isaacs JT, Dahiya R, Cunha GR. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ 8: 192–200, 2001. doi: 10.1038/sj.cdd.4400797. [DOI] [PubMed] [Google Scholar]
- 39.Kwong J, Xuan JW, Chan PSF, Ho S-M, Chan FL. A comparative study of hormonal regulation of three secretory proteins (prostatic secretory protein-PSP94, probasin, and seminal vesicle secretion II) in rat lateral prostate. Endocrinology 141: 4543–4551, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169–196, 2005. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 41.Lakshmanan MR, Chansang H, Olson JA. Purification and properties of carotene 15,15′-dioxygenase of rabbit intestine. J Lipid Res 13: 477–482, 1972. [PubMed] [Google Scholar]
- 42.Lakshmanan MR, Pope JL, Olson JA. The specificity of a partially purified carotenoid cleavage enzyme of rabbit intestine. Biochem Biophys Res Commun 33: 347–352, 1968. doi: 10.1016/0006-291X(68)90791-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee S-A, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW Jr, Harrison EH, Goldberg IJ, Maurer MS, Blaner WS. Cardiac dysfunction in β-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am J Physiol Heart Circ Physiol 307: H1675–H1684, 2014. doi: 10.1152/ajpheart.00548.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15′-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J 23: 1041–1053, 2009. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 45.Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the β-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr 142: 161S–165S, 2012. doi: 10.3945/jn.111.140756. [DOI] [PubMed] [Google Scholar]
- 46.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J Biol Chem 277: 23942–23948, 2002. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 47.Lindqvist A, Andersson S. Cell type-specific expression of β-carotene 15,15′-mono-oxygenase in human tissues. J Histochem Cytochem 52: 491–499, 2004. doi: 10.1177/002215540405200407. [DOI] [PubMed] [Google Scholar]
- 48.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 137: 2346–2350, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu C-H, Ford NA, Erdman JW Jr. Lycopene biodistribution is altered in 15,15′-carotenoid monooxygenase knockout mice. J Nutr 138: 2367–2371, 2008. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 275: 11915–11920, 2000. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Li W, Piao X, Zhang J, Zhang D, Wei N, Hu D, Liu S. Icariside II reduces testosterone production by inducing necrosis in rat Leydig cells. J Biochem Mol Toxicol 27: 243–250, 2013. doi: 10.1002/jbt.21481. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Wang D, Zhang J, Zhang D, Gong M, Wang C, Wei N, Liu W, Wang Y, Zhao C, Cui Y, Hu D. Citrinin reduces testosterone secretion by inducing apoptosis in rat Leydig cells. Toxicol In Vitro 26: 856–861, 2012. doi: 10.1016/j.tiv.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 53.Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 288: 9017–9027, 2013. doi: 10.1074/jbc.M112.444240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Love HD, Booton SE, Boone BE, Breyer JP, Koyama T, Revelo MP, Shappell SB, Smith JR, Hayward SW. Androgen regulated genes in human prostate xenografts in mice: relation to BPH and prostate cancer. PLoS One 4: e8384, 2009. doi: 10.1371/journal.pone.0008384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 22: 207–221, 2008. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Manna PR, Chandrala SP, Jo Y, Stocco DM. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37: 81–95, 2006. doi: 10.1677/jme.1.02065. [DOI] [PubMed] [Google Scholar]
- 57.O’Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis—androstenedione is the major androgen secreted by fetal/neonatal Leydig cells. Endocrinology 141: 2631–2637, 2000. doi: 10.1210/en.141.7.2631. [DOI] [PubMed] [Google Scholar]
- 58.O’Shaughnessy PJ. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol 29: 55–65, 2014. doi: 10.1016/j.semcdb.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Olson JA, Hayaishi O. The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci USA 54: 1364–1370, 1965. doi: 10.1073/pnas.54.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osborne JA. Estimating the false discovery rate using SAS [Online]. In: Proceedings of the Thirty-first Annual SAS Users Group International Conference. Chicago, IL: SAS Institute, 190–31, 2006, http://www2.sas.com/proceedings/sugi31/190-31.pdf. [Google Scholar]
- 61.Ouvrier A, Cadet R, Vernet P, Laillet B, Chardigny J-M, Lobaccaro J-MA, Drevet JR, Saez F. LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner. J Lipid Res 50: 1766–1775, 2009. doi: 10.1194/jlr.M800657-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J Biol Chem 276: 32,160–32,168, 2001. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- 63.Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J 28: 4457–4469, 2014. doi: 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25: 947–970, 2004. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 65.Payne AH. Steroidogenic enzymes in Leydig cells. In: Contemporary Endocrinology: The Leydig Cell in Health and Disease, edited by Payne AH, and Hardy MP. New York: Springer, 2007, p. 157–171. doi: 10.1007/978-1-59745-453-7_10 [DOI] [Google Scholar]
- 66.Perry JRB, Ferrucci L, Bandinelli S, Guralnik J, Semba RD, Rice N, Melzer D, Saxena R, Scott LJ, McCarthy MI, Hattersley AT, Zeggini E, Weedon MN, Frayling TM, DIAGRAM Consortium . Circulating β-carotene levels and type 2 diabetes-cause or effect? Diabetologia 52: 2117–2121, 2009. doi: 10.1007/s00125-009-1475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poliakov E, Gentleman S, Cunningham FX Jr, Miller-Ihli NJ, Redmond TM. Key role of conserved histidines in recombinant mouse β-carotene 15,15′-monooxygenase-1 activity. J Biol Chem 280: 29217–29223, 2005. doi: 10.1074/jbc.M500409200. [DOI] [PubMed] [Google Scholar]
- 68.Prince FP. The human Leydig cell. In: Contemporary Endocrinology: The Leydig Cell in Health and Disease, edited by Payne AH, and Hardy MP. New York: Springer, 2007, p. 71–89. [Google Scholar]
- 69.Qu Y, Huang X, Li Z, Liu J, Wu J, Chen D, Zhao F, Mu D. miR-199a-3p inhibits aurora kinase A and attenuates prostate cancer growth: new avenue for prostate cancer treatment. Am J Pathol 184: 1541–1549, 2014. doi: 10.1016/j.ajpath.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 70.Rajan P, Stockley J, Sudbery IM, Fleming JT, Hedley A, Kalna G, Sims D, Ponting CP, Heger A, Robson CN, McMenemin RM, Pedley ID, Leung HY. Identification of a candidate prognostic gene signature by transcriptome analysis of matched pre- and post-treatment prostatic biopsies from patients with advanced prostate cancer. BMC Cancer 14: 977, 2014. doi: 10.1186/1471-2407-14-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX Jr. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J Biol Chem 276: 6560–6565, 2001. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 72.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951, 1993. [DOI] [PubMed] [Google Scholar]
- 73.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell 9: 601–610, 2002. doi: 10.1016/S1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 75.Shiota M, Yokomizo A, Masubuchi D, Tada Y, Inokuchi J, Eto M, Uchiumi T, Fujimoto N, Naito S. Tip60 promotes prostate cancer cell proliferation by translocation of androgen receptor into the nucleus. Prostate 70: 540–554, 2010. doi: 10.1002/pros.21088. [DOI] [PubMed] [Google Scholar]
- 76.Stocco DM. The role of StAR in Leydig cell steroidogenesis. In: Contemporary Endocrinology: The Leydig Cell in Health and Disease, edited by Payne AH, and Hardy MP. New York: Springer, 2007, p. 149–155. doi: 10.1007/978-1-59745-453-7_9 [DOI] [Google Scholar]
- 77.Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 64: 479–498, 2002. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]
- 78.Terada N, Ogasawara Y, Yamane T, Matsumoto K, Kitamura Y. Heterogeneity in mouse seminal vesicle epithelial cells responding to androgen as evaluated by incorporation of [125I]iododeoxyuridine. Endocrinology 116: 1466–1472, 1985. doi: 10.1210/endo-116-4-1466. [DOI] [PubMed] [Google Scholar]
- 79.Tsai M-Y, Yeh S-D, Wang R-S, Yeh S, Zhang C, Lin H-Y, Tzeng C-R, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci USA 103: 18975–18980, 2006. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan L, Tan H-L, Thomas-Ahner JM, Pearl DK, Erdman JW Jr, Moran NE, Clinton SK. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev Res (Phila) 7: 1228–1239, 2014. doi: 10.1158/1940-6207.CAPR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang TTY, Edwards AJ, Clevidence BA. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to β-carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms. J Nutr Biochem 24: 1538–1546, 2013. doi: 10.1016/j.jnutbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Wang X-D, Wang B-E, Soriano R, Zha J, Zhang Z, Modrusan Z, Cunha GR, Gao W-Q. Expression profiling of the mouse prostate after castration and hormone replacement: implication of H-cadherin in prostate tumorigenesis. Differentiation 75: 219–234, 2007. doi: 10.1111/j.1432-0436.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 83.Warden CH, Langner CA, Gordon JI, Taylor BA, McLean JW, Lusis AJ. Tissue-specific expression, developmental regulation, and chromosomal mapping of the lecithin: cholesterol acyltransferase gene. Evidence for expression in brain and testes as well as liver. J Biol Chem 264: 21573–21581, 1989. [PubMed] [Google Scholar]
- 84.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schröder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci 50: 857–861, 1992. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 85.Widjaja-Adhi MAK, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet 24: 3206–3219, 2015. doi: 10.1093/hmg/ddv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson JD. The critical role of androgens in prostate development. Endocrinol Metab Clin North Am 40: 577–590, 2011. doi: 10.1016/j.ecl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Wu Z, Cho H, Hampton GM, Theodorescu D. Cdc6 and cyclin E2 are PTEN-regulated genes associated with human prostate cancer metastasis. Neoplasia 11: 66–76, 2009. doi: 10.1593/neo.81048. [DOI] [PMC free article] [PubMed] [Google Scholar]