Abstract
Sympathetic outflow is modified during acute homeostatic stress through increased firing of low-threshold axons, recruitment of latent axons, and synaptic delay modifications. However, the role of central mechanisms versus peripheral reflex control over sympathetic recruitment remains unknown. Here, we examined sympathetic discharge patterns during fatiguing static handgrip (SHG) exercise and postexercise circulatory occlusion (PECO) to study the central vs. peripheral reflex elements of sympathetic neural coding. Muscle sympathetic nerve activity (MSNA; microneurography) was measured in six males (25 ± 3 yr) at baseline (3 min) and during 5 min of SHG exercise completed at 20% maximal voluntary contraction. Isolation of the peripheral metaboreflex component was achieved by PECO for 3 min. Action potential (AP) patterns were studied using wavelet-based methodology. Compared with baseline, total MSNA increased by minute 3 of SHG, remaining elevated throughout the duration of exercise and PECO (all P < 0.05). The AP content per burst increased above baseline by minute 4 of SHG (Δ4 ± 2), remaining elevated at minute 5 (Δ6 ± 4) and PECO (Δ4 ± 4; all P < 0.05). Similarly, total AP clusters increased by minute 4 of SHG (Δ5 ± 5) and remained elevated at minute 5 (Δ6 ± 3) and PECO (Δ7 ± 5; all P < 0.01), indicating recruitment of latent subpopulations. Finally, the AP cluster size-latency profile was shifted downward during minutes 4 (−32 ± 22 ms) and 5 (−49 ± 17 ms; both P < 0.05) of SHG but was not different than baseline during PECO (P > 0.05). Our findings suggest that central perceptual factors play a specific role in the synaptic delay aspect of sympathetic discharge timing, whereas peripheral reflex mechanisms affect recruitment of latent axons.
Keywords: action potential, exercise pressor reflex, metaboreflex, microneurography, sympathetic nerve activity
neural coding patterns represent a fundamental feature in the exchange and processing of neural information. Recently, we have confirmed that neural coding principals operate within the sympathetic nervous system (2, 3, 6, 29, 30, 36). Specifically, increases in muscle sympathetic nerve traffic in response to acute physiological stress are the result of increased firing of low-threshold axons (i.e., rate coding), as well as the recruitment of latent subpopulations of larger sized axons that are otherwise silent at baseline (i.e., population coding) (2, 3, 6, 29, 36). Furthermore, it appears that synaptic delay variations exist as a third strategy to alter autonomic outflow (2, 3, 30). These strategies have been confirmed in two fundamental reflexes; that is, the arterial baroreflex, studied during −80 mmHg lower body negative pressure (LBNP) (3, 29) and the Valsalva maneuver (30), as well as the arterial chemoreflex, as inferred during maximal voluntary apnea (2, 3, 6, 36). However, a persistent question remains, particularly with the apnea response, as to whether or not these recruitment strategies are restricted to the perception of effort during severe stress scenarios (i.e., a central command phenomenon), or to the severe reflex stress alone (i.e., a peripheral reflex mechanism). Evidence from the Valsalva maneuver and voluntary apnea data do not exclude a role for a central perception of effort determinant, whereas the LBNP data suggest a dominant role for peripheral reflex mechanisms. Additionally, one feature that may especially relate to central vs. peripheral features is the ability to acutely modify reflex latency. That is, compared with resting levels, neural activation latency was reduced during voluntary apnea (2, 3) and the Valsalva maneuver (30) but increased during LBNP-induced baroreceptor unloading (3). As such, the exact central vs. peripheral mechanisms of these sympathetic neural recruitment strategies remain to be fully elucidated.
The homeostatic response to fatiguing exercise represents a unique reflex in which the central and peripheral elements of the reflex can be studied separately as they develop over time. Specifically, alterations in efferent neural outflow during exercise are mediated through a highly coordinated and integrative system of reflexes involving feed-forward signals emanating from higher brain centers (i.e., central command) (10, 14, 16), as well as peripheral feedback from type III and IV muscle sensory afferents within active skeletal muscle (i.e., exercise pressor reflex) (1, 15, 20, 21). The exercise pressor reflex elicits large increases in muscle sympathetic nerve activity (MSNA) (19, 27, 32, 33, 35, 39, 41), albeit after a workload-dependent delay of ~30 to 60 s (19). Sympathetic activity remains elevated during postexercise circulatory occlusion (PECO), which suggests that the mechanism of activation relates to muscle metabolism and a dominant metaboreflex component. Furthermore, a marked increase in integrated sympathetic burst size characterizes this reflex, most likely reflective of increases in the number (25) and/or size (29) of action potentials (APs) firing more or less at the same time. Recently, Murai et al. (24) examined the firing patterns of a single axon that was present at baseline and sustained during static handgrip (SHG) exercise and found elevations in total sympathetic activity but no change in the probability of multiple within-burst firing of that axon. Combined, the observations of no change in the probability of a single axon firing, but the need for additional and/or larger axons to account for larger bursts, suggest that latent axons are recruited in the large sympathetic response to fatiguing exercise. Thus fatiguing exercise and the muscle metaboreflex represent a unique model to study the central vs. peripheral features of sympathetic neural recruitment, as it allows the opportunity to isolate the peripheral reflex component from the central component (1).
Therefore, to study the varying roles played by central vs. peripheral elements in the neural coding strategies used by the sympathetic nervous system during acute physiological stress, we used a multiunit AP approach to examine sympathetic outflow patterns during fatiguing SHG exercise and a period of PECO. The latter was designed to maintain and isolate the muscle metaboreflex (i.e., peripheral reflex component) from the central command features of the reflex (1).
METHODS
Participants.
Six young healthy, normotensive males (25 ± 3 yr, 179 ± 7 cm, 82 ± 6 kg; body mass index = 26 ± 2 kg/m2) were studied after providing informed written consent. Participants were recreationally active, nonsmokers, and otherwise healthy with no history of cardiovascular or respiratory disease. The study was approved by the Health Sciences Research Ethics Board at Western University in London, Canada.
Experimental protocol.
Participants were tested following a 3-h fast, a 12-h abstinence from caffeinated products and alcohol, and a 24-h abstinence from strenuous exercise. Testing was completed in the supine position. Participants performed two maximal voluntary contractions (MVCs) with their nondominant hand (left hand in all participants), and the largest of the two was used to calculate relative handgrip intensity for the ensuing SHG protocol. After a 3-min baseline rest period, participants performed SHG exercise for 5 min at 20% MVC, which was followed immediately by PECO for 3 min. PECO was initiated ~5 s before exercise completion by rapidly inflating a pneumatic cuff (Hokanson SC12D; D. E. Hokanson, Bellevue, WA) on the upper exercised arm to suprasystolic levels (~200 mmHg). Throughout SHG, visual feedback of exercise intensity was provided to aid participants in the maintenance of target force. Upon exercise completion, and before PECO onset, participants assessed their level of perceived exertion using the 20-point Borg Rating of Perceived Exertion Scale (5).
Experimental measures.
Sympathetic neural recordings were obtained in the right peroneal nerve using microneurography (11) (662C-3; Bioengineering of University of Iowa, Iowa City, IA). The methodology used in our laboratory has been described previously in detail (3, 29, 36). Heart rate (HR) was determined throughout the study protocol from a standard three-lead electrocardiogram. Mean arterial pressure (MAP) was obtained on a beat-to-beat basis using finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) on the nonexercised hand, which was calibrated to manual sphygmomanometry values. The Finometer Modelflow algorithm was used to calculate stroke volume (SV) and cardiac output (CO), and total peripheral resistance (TPR) was calculated as MAP divided by CO. Data were collected using LabChart7 and PowerLab (ADInstruments, Colorado Springs, CO).
Data analysis.
Data were analyzed for the 3-min baseline period, for each minute of the 5-min SHG exercise, and for the 3-min PECO period. Sympathetic bursts were identified from the integrated MSNA neurogram if 1) they exhibited pulse synchrony; 2) they had a signal-to-noise ratio of at least 2:1 with respect to the previous period of neural silence between bursts; 3) they presented with characteristic rising and falling slopes; and 4) APs were visible in the corresponding raw and filtered neurograms. The level of integrated sympathetic activity was assessed using burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats). Furthermore, burst amplitude, which reflects both the size (29) and number (25) of APs recruited within each burst, was assessed. However, due to the potential influence of microelectrode placement on the amplitude of the integrated signal (37, 38), burst amplitude was normalized within each individual to the largest burst at baseline, which was assigned a value of 100 arbitrary units (AU). Finally, total MSNA (product of burst frequency and normalized burst amplitude) was determined.
Sympathetic APs were extracted from the filtered raw MSNA signal using wavelet-based methodology, as first used by others (7, 9), and modified in our laboratory (28). Briefly, a continuous wavelet transform with a “mother wavelet” (adapted from actual physiological recordings of postganglionic sympathetic APs) was applied to the filtered raw MSNA signal to extract sympathetic APs at their point of incidence. Extracted APs were ordered based on their peak-to-peak amplitude, and histogram analysis was conducted to separate APs into ‘clusters’ (i.e., bins of similarly sized APs) (31). The AP detection, extraction, and classification process, as well as the reliability and repeatability of our AP detection technique, has been described previously in detail, and readers are directed to this previous work (3, 28, 36). Next, within participants, cluster characteristics were normalized to ensure that bin width, maximum bin center, and the total number of bins would be identical across conditions (i.e., baseline to SHG to PECO). This normalization procedure ensured that corresponding clusters across conditions contain APs with similar peak-to-peak amplitudes. Therefore, an increase in total clusters during SHG or PECO represents recruitment of subpopulations of previously silent, larger sized AP families that were not present at baseline. Finally, the average signal-to-noise ratio in the current study was 4.4 ± 0.6, and based on previous validation analysis of our analytical technique (28), this is expected to produce a correct detection rate of >90% and a false positive rate of <3%.
Patterns of AP activity were expressed using AP frequency (APs/min). Recruitment of sympathetic APs was quantified using the mean AP content per integrated sympathetic burst (APs/burst), the number of total AP clusters, and the number of active AP clusters per integrated sympathetic burst (clusters/burst). AP cluster latency was calculated as the mean AP latency of all APs present within that respective cluster, which was measured as the time delay between the R-wave of the preceding cardiac cycle and the negative peak of the AP waveform. As the number of total AP clusters varied between participants, the number of total clusters was normalized to 10 clusters, each containing 10% ranges of the largest detected cluster, which was given a value of 100% (i.e., 0–10%, 10–20%, etc.), when assessing the AP cluster size-latency relationship (2, 3).
Statistical analyses.
One-way repeated-measures ANOVAs assessed the effect of time (i.e., baseline to SHG to PECO) on each hemodynamic, integrated MSNA, and AP variable (SigmaPlot 12.0; Systat Software, San Jose, CA). Data were tested for normal distribution using Shapiro-Wilk tests. Non-normally distributed data were assessed using Friedman repeated measures ANOVAs on ranks. Significant main effects were assessed using Tukey’s post hoc analysis. Statistical significance was set at P < 0.05 and all data are presented as means ± SD.
RESULTS
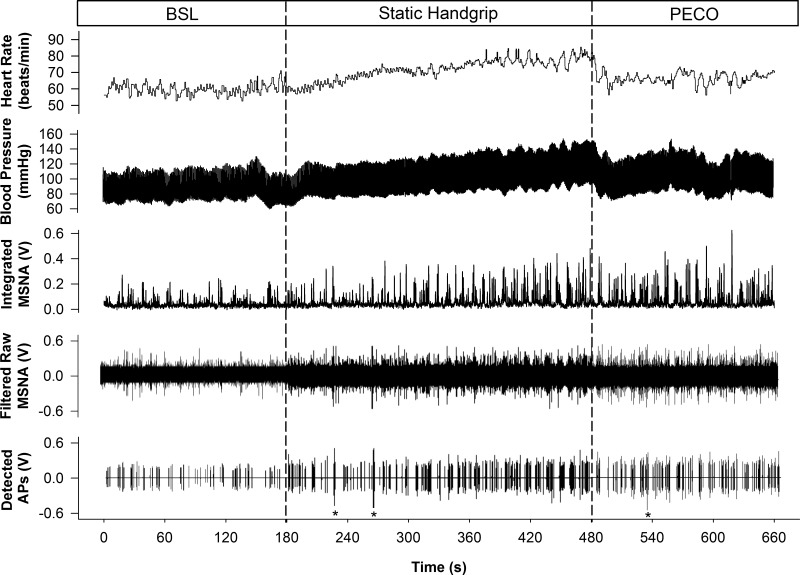
Figure 1 displays original recordings of HR, MAP, integrated MSNA, filtered raw MSNA, and detected APs from one participant. On average, SHG exercise was completed at 20 ± 1% MVC and participants reported a Borg scale rating of 17 ± 2 at the end of exercise.
Fig. 1.
Representative sample of data collected from 1 subject at baseline (BSL) and during static handgrip exercise and postexercise circulatory occlusion (PECO). HR, heart rate; BP, blood pressure; MSNA, muscle sympathetic nerve activity; AP, action potential. *Denotes noise spikes not included in analysis.
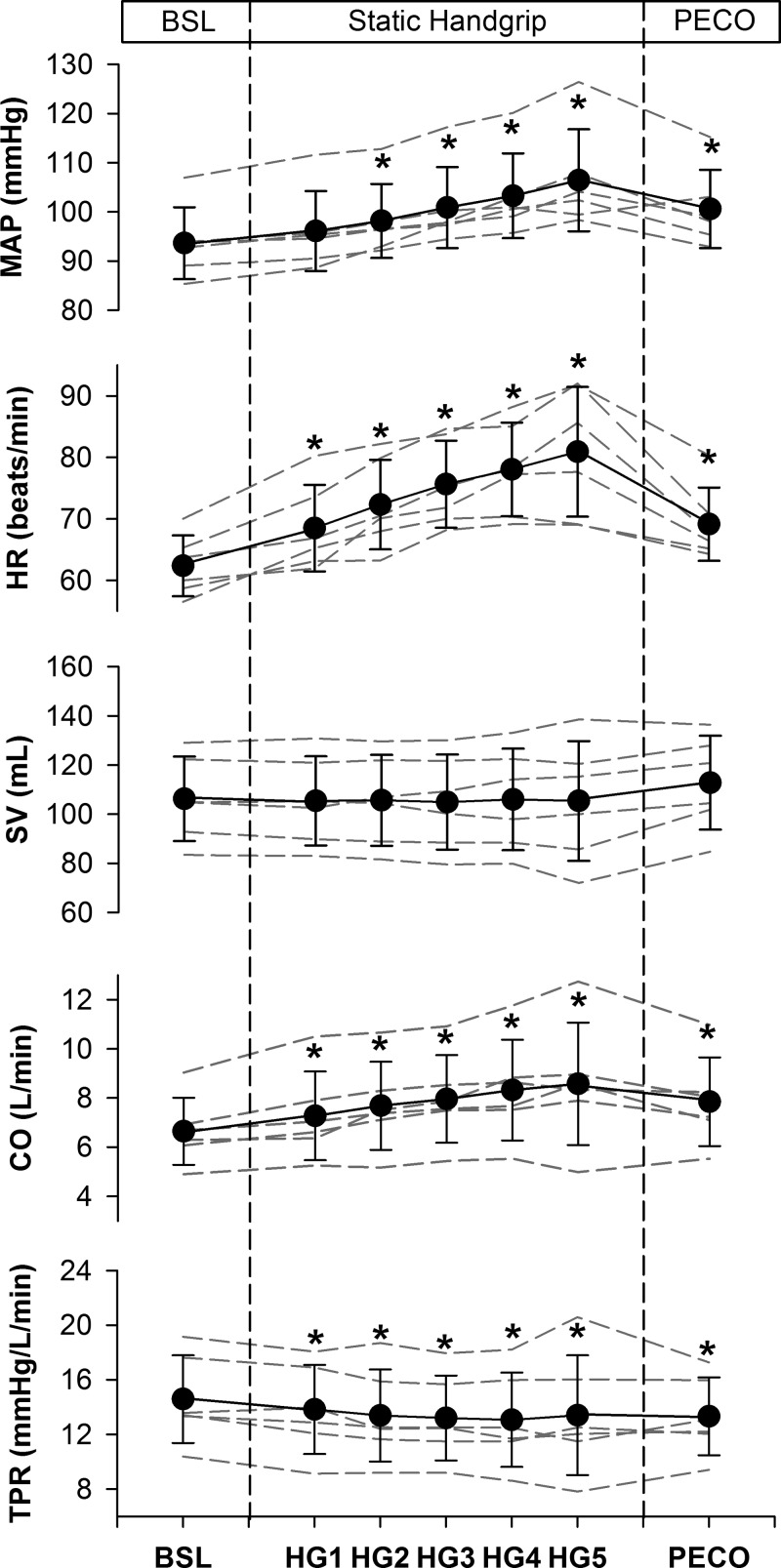
Figure 2 displays the mean and individual hemodynamic responses to SHG and PECO and Table 1 provides the delta values on going from baseline to each period of the SHG and PECO protocol. At baseline, MAP was 94 ± 7 mmHg, HR was 62 ± 5 beats/min, SV was 106 ± 17 ml, CO was 6.7 ± 1.4 l/min, and TPR was 15 ± 3 mmHg·l−1·min. When compared with baseline, MAP was increased by minute 2 of SHG, and remained elevated throughout the duration of exercise and PECO (all P < 0.01). HR was elevated during the entire SHG period and remained elevated during PECO (all P < 0.001), but at levels that were less than those observed during minutes 3 to 5 of SHG (all P < 0.05). When compared with baseline, SV was unchanged during SHG and PECO (all P > 0.05). CO was elevated throughout SHG and PECO (all P < 0.05). Finally, compared with baseline, TPR was reduced throughout SHG and PECO (all P < 0.05).
Fig. 2.
Hemodynamic responses to static handgrip (HG) exercise and postexercise circulatory occlusion (PECO). Dashed lines represent individual data. Values are means ± SD. MAP, mean arterial pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance. *P < 0.05, significantly different from baseline (BSL).
Table 1.
Hemodynamic responses to SHG and PECO
| Static Handgrip |
PECO | |||||
|---|---|---|---|---|---|---|
| HG1 | HG2 | HG3 | HG4 | HG5 | ||
| ΔMAP, mmHg | 2 ± 1 | 5 ± 2* | 7 ± 4* | 10 ± 5* | 13 ± 7* | 7 ± 4* |
| ΔHR, beats/min | 6 ± 3* | 10 ± 4* | 13 ± 3* | 16 ± 3* | 19 ± 6* | 7 ± 2* |
| ΔSV, ml | −1 ± 2 | −1 ± 2 | −1 ± 4 | 0 ± 6 | −1 ± 9 | 7 ± 6 |
| ΔCO, l/min | 0.6 ± 0.5* | 1.0 ± 0.5* | 1.3 ± 0.5* | 1.7 ± 0.8* | 1.9 ± 1.2* | 1.2 ± 0.6* |
| ΔTPR, mmHg·l−1·min | −0.8 ± 0.7* | −1.2 ± 0.5* | −1.4 ± 0.5* | −1.5 ± 0.5* | −1.2 ± 1.4* | −1.3 ± 0.6* |
Values are means ± SD. SHG, static handgrip; PECO, postexercise circulatory occlusion; HG, handgrip; MAP, mean arterial pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance.
P < 0.05, significantly different from baseline.
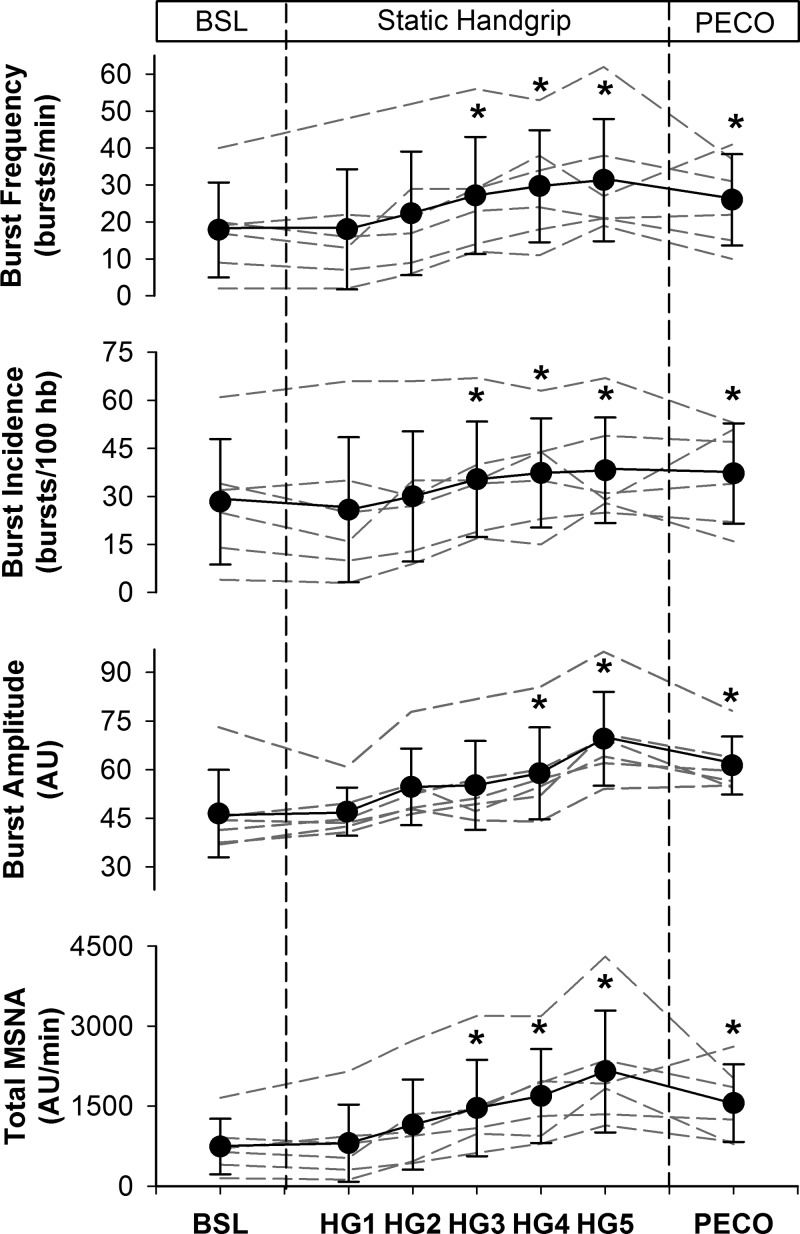
Figure 3 illustrates the mean and individual integrated MSNA responses to SHG and PECO and Table 2 provides the delta values. At baseline, burst frequency was 18 ± 13 bursts/min, burst incidence was 28 ± 20 bursts/100 heartbeats, burst amplitude was 46 ± 13 AU, and total MSNA was 742 ± 519 AU/min. When compared with baseline, burst frequency and burst incidence were increased by minute 3 of SHG exercise and remained elevated for the duration of exercise and PECO (all P < 0.05). Furthermore, burst amplitude increased above baseline by minute 4 of SHG and remained elevated at minute 5 and during PECO (all P < 0.01). Finally, total MSNA increased by minute 3 of SHG, remaining elevated above baseline levels for the duration of exercise and PECO (all P < 0.05).
Fig. 3.
Integrated muscle sympathetic nerve activity (MSNA) responses to static handgrip (HG) exercise and postexercise circulatory occlusion (PECO). Dashed lines represent individual data. Values are means ± SD. AU, arbitrary units; hb, heartbeats. *P < 0.05, significantly different from baseline (BSL).
Table 2.
Integrated MSNA and action potential responses to SHG and PECO
| Static Handgrip |
PECO | |||||
|---|---|---|---|---|---|---|
| HG1 | HG2 | HG3 | HG4 | HG5 | ||
| Integrated MSNA | ||||||
| ΔBF, bursts/min | 0 ± 5 | 5 ± 6 | 9 ± 5* | 12 ± 6* | 14 ± 8* | 8 ± 9* |
| ΔBI, bursts/100 heartbeats | −3 ± 6 | 2 ± 6 | 7 ± 5* | 9 ± 7* | 10 ± 10* | 9 ± 12* |
| ΔBA, AU | 1 ± 7 | 8 ± 3 | 9 ± 7 | 12 ± 8* | 23 ± 8* | 15 ± 8* |
| ΔTotal MSNA, AU/min | 64 ± 248 | 412 ± 407* | 724 ± 496* | 948 ± 489* | 1,407 ± 786* | 814 ± 646* |
| AP indexes | ||||||
| ΔAPs/min | 44 ± 92 | 139 ± 187* | 197 ± 201* | 252 ± 203* | 318 ± 215* | 195 ± 296* |
| ΔAPs/burst | 1 ± 1 | 2 ± 2 | 3 ± 3 | 4 ± 2* | 6 ± 4* | 4 ± 4* |
| ΔClusters/burst | 0.7 ± 0.4 | 0.8 ± 0.7 | 1.2 ± 0.8 | 1.4 ± 0.7* | 1.9 ± 1.3* | 1.3 ± 1.0* |
| ΔTotal clusters | 0 ± 3 | 1 ± 3 | 2 ± 4 | 5 ± 5* | 6 ± 3* | 7 ± 5* |
Values are means ± SD. MSNA, muscle sympathetic nerve activity; SHG, static handgrip; PECO, postexercise circulatory occlusion; HG, handgrip; BF, burst frequency; BI, burst incidence; BA, burst amplitude; AU, arbitrary units; AP, action potential.
P < 0.05, significantly different from baseline.
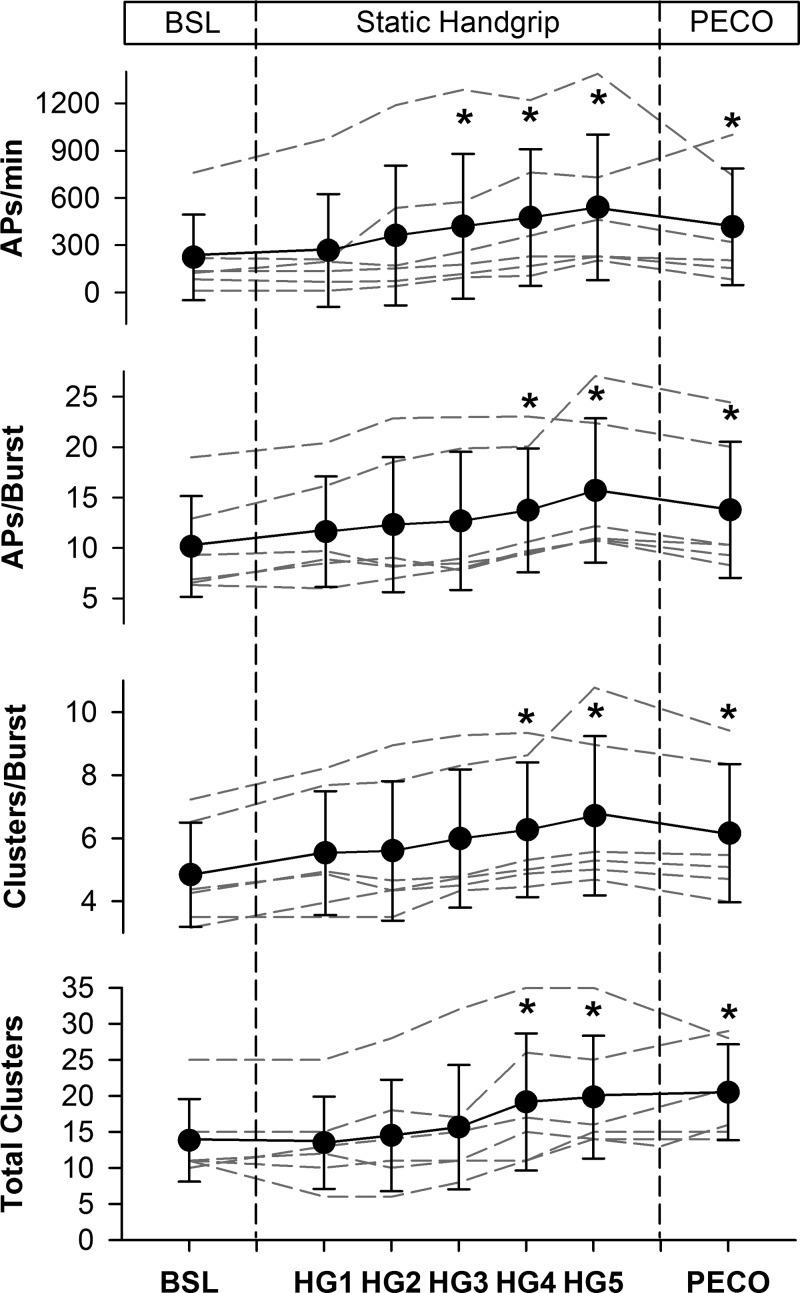
Figure 4 outlines the mean and individual AP responses to SHG and PECO and Table 2 provides the delta values. At baseline, AP frequency was 223 ± 271 APs/min, the mean AP content per burst was 10 ± 5 APs/burst, the number of active clusters per burst was 5 ± 2, and the number of total clusters was 14 ± 6. When compared with baseline, AP frequency increased by minute 3 of SHG and remained elevated throughout SHG and PECO (all P < 0.05). The mean AP content per integrated sympathetic burst increased by minute 4 of SHG and remained elevated at minute 5 and PECO (all P < 0.05). Similarly, the number of active clusters per integrated burst was elevated by minute 4 and stayed elevated throughout the remainder of SHG and PECO (all P < 0.01). Finally, compared with baseline, the number of total AP clusters was increased at minutes 4 and 5 of SHG, and remained elevated during PECO (all P < 0.01).
Fig. 4.
Sympathetic action potential (AP) responses to static handgrip (HG) exercise and postexercise circulatory occlusion (PECO). Dashed lines represent individual data. Values are means ± SD. *P < 0.05, significantly different from baseline (BSL).
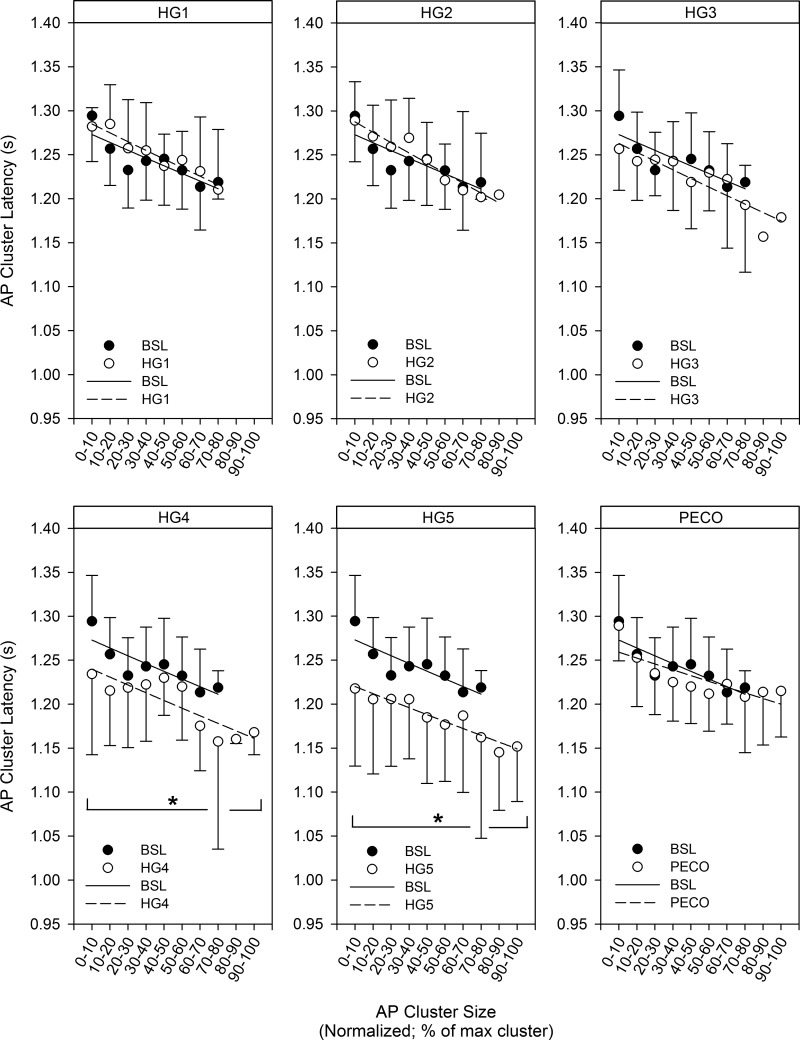
At baseline, AP cluster latency decreased as a function of normalized cluster number (i.e., as the peak-to-peak AP cluster amplitude increased), such that larger-sized APs exhibited shorter reflex latency. The mean response was fitted using an exponential function model (R2 = 0.73; P < 0.001). The same pattern was observed throughout SHG exercise (HG1: R2 = 0.92; HG2: R2 = 0.93; HG3: R2 = 0.83; HG4: R2 = 0.69; and HG5: R2 = 0.91; all P < 0.01) and PECO (R2 = 0.65; P < 0.01). When compared with baseline, the AP cluster size-latency profile for corresponding clusters was not shifted significantly upward or downward during the first 3 min of SHG exercise (HG1: mean = 8 ± 16 ms, range −12 to 28 ms; HG2: mean = 4 ± 17 ms, range −17 to 27 ms; and HG3: mean = −11 ± 18 ms, range −38 to 12 ms; all P > 0.05). However, compared with baseline, the AP cluster size-latency profile was shifted downward for every corresponding cluster during minutes 4 (mean = −32 ± 22 ms, range −5 to −61 ms; P < 0.05) and 5 (mean = −49 ± 17 ms, range −27 to −77 ms; P < 0.05; Fig. 5) of SHG exercise, such that all similarly sized APs were recruited earlier during SHG. During PECO, the AP cluster size-latency profile was not different than baseline levels (mean = −9 ± 12 ms, range −25 to 9 ms; P > 0.05).
Fig. 5.
Action potential (AP) cluster latency as a function of AP cluster size at baseline (BSL), minutes 1-5 of static handgrip (HG), and postexercise circulatory occlusion (PECO). No significant upward or downward shift was seen in the AP cluster size-latency profile during HG1 (range −14 to 36 ms; range represents individual data), HG2 (range −18 to 35 ms), or HG3 (range −53 to 10 ms), whereas a significant downward shift in the profile was observed during HG4 (range 4 to −136 ms) and HG5 (range −9 to −198 ms). Finally, no significant upward or downward shift was seen during PECO (range −28 to 22 ms). *P < 0.05, significantly different from BSL.
DISCUSSION
This study examined AP recruitment patterns during the SHG and PECO phases of the exercise pressor response maneuver to gain insight into the role that central command elements versus peripheral reflex mechanisms have in AP recruitment and sympathetic outflow patterns in humans. The major findings of the current study are as follows: 1) In response to exercise pressor reflex activation, the sympathetic nervous system increases efferent nerve discharge through increased firing of previously recruited axons and also through the recruitment of latent subpopulations of larger-sized, faster conducting axons. These larger and faster APs were present at the end of the SHG period and persisted throughout the PECO phase. 2) The latency of all APs was reduced towards the end of fatiguing SHG exercise but not during the PECO phase. The results suggest that central command features may play a specific role in modifying the synaptic delay aspect of efferent sympathetic discharge timing, whereas peripheral muscle chemoreflex mechanisms affect the recruitment of latent high-threshold APs.
Fatiguing SHG exercise elicits robust increases in muscle sympathetic outflow through activation of mechanically and metabolically sensitive type III and IV sensory afferents (15, 21) and perhaps modulatory input from higher brain sites during more intense contraction (40). To date, quantification of the integrated MSNA neurogram has formed the basis of determining the sympathetic response to this reflex, with the finding that MSNA is increased after an initial delay (19), the timing of which is most likely influenced inversely by contraction intensity (12). Indeed, in the current study, MSNA burst frequency and incidence were elevated after ~2 min of SHG exercise completed at the relatively modest intensity of 20% MVC.
Presently, using a multiunit AP approach, we show for the first time that the sympathetic nervous system responds to fatiguing SHG exercise by increasing the firing of already recruited lower threshold axons and through the recruitment of subpopulations of previously silent (i.e., not present at baseline), higher threshold and faster conducting (and therefore, larger) axons. This is similar to patterns observed previously during both baroreflex (3, 29) and arterial chemoreflex (2, 3, 6, 36) stimuli and is supported by the concept that C-fiber diameter variations (~0.5 to 2.0 µm) exist within the human peroneal nerve (37). Since these larger and faster APs became evident after 3 min of exercise, and persisted throughout the PECO period, their recruitment appears to be related to peripheral events within the muscle and, therefore, related to the metaboreflex. These observations discount a role for central factors in eliciting the recruitment of these latent fibers, at least in this reflex model. Interestingly, the different time course of change between integrated MSNA indexes (i.e., increased after 2 min) and AP recruitment (i.e., increased after 3 min) may lend important information as to the order of discharge options used by the sympathetic nervous system. Specifically, MSNA burst frequency increased before the number of APs per burst or total AP clusters increased, which suggests that the first option for the sympathetic nervous system is to increase firing frequency and the second option is recruitment of additional fibers, as previously hypothesized (17), and similar to the ordered recruitment observed in the motor system (i.e., Henneman's Size Principle) (13). This also supports the conjecture that these axons are reserved for periods of high or severe physiological stress (3, 29). In line with this, recruitment of new, larger axons was observed in all individuals during −80 mmHg LBNP but in only ~30% of these same individuals during −60 mmHg LBNP (29). Taken together, it appears as though a fixed, reflex-independent recruitment strategy exists within the sympathetic nervous system, whereby subpopulations of larger neurons are recruited during severe stress scenarios requiring high sympathetic outflow, albeit with some interindividual variations. Furthermore, the current results suggest that peripheral reflex mechanisms play a dominant role in mediating this recruitment response. Whether this conclusion applies across all reflexes will require additional study. However, central command likely has a small role in LBNP, lending further support to the reflexive role played by peripheral sensory mechanisms in AP recruitment.
In addition to the above-described recruitment options, the sympathetic nervous system also modifies efferent firing of lower threshold axons through acute variations of reflex activation latency (2, 3, 30). In further support of this, we show here that the AP cluster size-latency profile was shifted downwards toward the end of fatiguing SHG exercise, such that during minutes 4 and 5 of SHG, all APs expressed a shorter latency. Similar observations have been made during the Valsalva maneuver (30) and during maximal voluntary apnea (2, 3). That this downward shift occurred during both peripheral chemoreflex activation (i.e., during volitional apnea) (2, 3) and muscle chemoreflex activation (i.e., during SHG) suggests perhaps that chemoreflex stress may be involved. However, during isolation of the metaboreflex with PECO in the current study, the AP cluster size-latency profile was shifted back toward baseline resting levels, indicating that the muscle chemosensitive sensory afferents are unlikely to play a role in our observed findings. Rather, the common pattern of a downward shift in the AP cluster size-latency profile during the Valsalva maneuver (30), during maximal voluntary apnea (2, 3), and toward the end of fatiguing SHG exercise here, suggests that a perceptual component (i.e., central command) may be involved in this outcome. Specifically, each maneuver exhibits a certain level of volitional stress that is related to individual tolerance and perception of the maneuver. The exact mechanism(s)/source(s) for this change in AP latency cannot be discerned from the current analysis and requires additional study. However, in as much as conduction velocity of a given axon is relatively stable, our observations suggest the possibility of variations in synaptic delays somewhere within the neural pathway. Indeed, Macefield et al. (18) noted that the AP latency of a single axon can vary rather significantly (i.e., 358 ± 33 ms), demonstrating the potential for considerable modification of synaptic delays and/or central processing times. The functional significance of this systemic and coordinated shift to shorter reflex latency remains unknown. However, the consistent observation of this downward shift in latency during several sympathoexcitatory maneuvers suggests it may be a response reserved for severe stress scenarios requiring high, and perhaps faster occurring, sympathetic outflow. Nonetheless, it appears that acute malleability of synaptic delays and/or central processing times may represent a unique option available to alter efferent sympathetic outflow during fatiguing SHG exercise, and this is perhaps mediated through a central command phenomenon.
While the use of fatiguing SHG exercise and PECO offers a unique model to study the central versus peripheral features of sympathetic neural recruitment, we cannot conclude with certainty that the PECO phase represents solely peripheral (i.e., metaboreflex) feedback to the central nervous system, without any lingering central, or perceptual, component. Indeed, the discomfort experienced during this period, and retained pressor response, may sustain and/or modify residual centrally determined sympathetic patterns. In this sense, the lack of a control exercise recovery (i.e., no PECO phase following SHG exercise) period, as performed by other groups (8, 22), may represent a limitation of the current study. However, data from these groups using a handgrip model without PECO support the concept that the occlusion phase represents selectively peripheral, metaboreflex-mediated input, as the exercise pressor response is maintained during PECO but absent during the control recovery phase (8). Furthermore, a “resting” circulatory occlusion period (i.e., not preceded by exercise) had no effect on baseline resting cardiovascular parameters, further discounting a role for central, or perceptual, elements during our PECO phase in the current study.
In summary, alterations in efferent sympathetic neural outflow during fatiguing exercise occur as a result of increased firing frequency and probability of lower threshold axons, as a result of recruitment of previously silent subpopulations of larger-sized, faster conducting axons, and perhaps through acute changes of synaptic delays and/or central processing times. Taken together, it appears as though this represents a generalized central neural coding response to severe stress within the sympathetic nervous system. Our findings here, although in one reflex model, suggest a role for central command features in mediating the synaptic delay aspect of sympathetic discharge patterning, whereas peripheral reflex mechanisms appear largely responsible for recruitment of latent sympathetic fibers.
Perspectives and Significance
The current work and analytical technique complements traditional integrated methodology but provides a novel perspective on sympathetic discharge patterns during exercise pressor reflex activation and, therefore, may offer new insights into important unanswered concepts, such as 1) the influence of these neural coding patterns on neurotransmitter release and end-organ vascular responses; 2) the apparent decoupling between elevated sympathetic outflow and vascular resistance in the inactive limb during metaboreflex activation (i.e., neurovascular transduction) (34); 3) the complex interplay between and neurocircuitry involved in central command and perception of effort during exercise and the potential for feedback-related central control (4, 42); and 4) the exaggerated sympathetic responses to exercise (and in turn, limited exercise capacity) observed in certain clinical pathologies (i.e., congestive heart failure) (23, 26).
GRANTS
This study was supported by funding from the Natural Sciences and Engineering Research Council of Canada Grant No. 217916–2013 (to J. K. Shoemaker), an Ontario Graduate Scholarship (to T. D. Olver), and a Canadian Institutes of Health Research Doctoral Research Award (to M. B. Badrov). J. K. Shoemaker is a Tier 1 Canada Research Chair.
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
M.B.B., T.D.O., and J.K.S., conception and design of research; M.B.B., T.D.O., and J.K.S. performed experiments; M.B.B., analyzed data; M.B.B., T.D.O., and J.K.S., interpreted results of experiments; M.B.B. and J.K.S., drafted manuscript; M.B.B., T.D.O., and J.K.S., edited and revised manuscript; M.B.B., T.D.O., and J.K.S., approved final version of manuscript.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badrov MB, Lalande S, Olver TD, Suskin N, Shoemaker JK. Effects of aging and coronary artery disease on sympathetic neural recruitment strategies during end-inspiratory and end-expiratory apnea. Am J Physiol Heart Circ Physiol 311: H1040–H1050, 2016. doi: 10.1152/ajpheart.00334.2016. [DOI] [PubMed] [Google Scholar]
- 3.Badrov MB, Usselman CW, Shoemaker JK. Sympathetic neural recruitment strategies: responses to severe chemoreflex and baroreflex stress. Am J Physiol Regul Integr Comp Physiol 309: R160–R168, 2015. doi: 10.1152/ajpregu.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol 97: 29–38, 2012. doi: 10.1113/expphysiol.2011.060848. [DOI] [PubMed] [Google Scholar]
- 5.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 6.Breskovic T, Steinback CD, Salmanpour A, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic neurons during breath-holding at different lung volumes in apnea divers and controls. Auton Neurosci 164: 74–81, 2011. doi: 10.1016/j.autneu.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Brychta RJ, Shiavi R, Robertson D, Diedrich A. Spike detection in human muscle sympathetic nerve activity using the kurtosis of stationary wavelet transform coefficients. J Neurosci Methods 160: 359–367, 2007. doi: 10.1016/j.jneumeth.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 9.Diedrich A, Charoensuk W, Brychta RJ, Ertl AC, Shiavi R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng 50: 41–50, 2003. doi: 10.1109/TBME.2002.807323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. doi: 10.1111/j.1365-201X.1968.tb10904.x. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto I, Miyamura M, Saito M. Initiation of increase in muscle sympathetic nerve activity delay during maximal voluntary contraction. Acta Physiol Scand 164: 293–297, 1998. doi: 10.1046/j.1365-201X.1998.00437.x. [DOI] [PubMed] [Google Scholar]
- 13.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965. [DOI] [PubMed] [Google Scholar]
- 14.Johansson JE. Ueber die Einwirkung der Muskelthatigkeit auf die Athmung und die Hertzthatigkeit. Skand Arch Physiol 5: 20–66, 1893. doi: 10.1111/j.1748-1716.1894.tb00192.x. [DOI] [Google Scholar]
- 15.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47: 112–136, 1913. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol 516: 293–301, 1999. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481: 799–809, 1994. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 22.Mulliri G, Sainas G, Magnani S, Palazzolo G, Milia N, Orrù A, Roberto S, Marongiu E, Milia R, Crisafulli A. Ischemic preconditioning reduces hemodynamic response during metaboreflex activation. Am J Physiol Regul Integr Comp Physiol 310: R777–R787, 2016. doi: 10.1152/ajpregu.00429.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murai H, Takamura M, Maruyama M, Nakano M, Ikeda T, Kobayashi D, Otowa K, Ootsuji H, Okajima M, Furusho H, Takata S, Kaneko S. Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol 587: 2613–2622, 2009. doi: 10.1113/jphysiol.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murai H, Takata S, Maruyama M, Nakano M, Kobayashi D, Otowa K, Takamura M, Yuasa T, Sakagami S, Kaneko S. The activity of a single muscle sympathetic vasoconstrictor nerve unit is affected by physiological stress in humans. Am J Physiol Heart Circ Physiol 290: H853–H860, 2006. doi: 10.1152/ajpheart.00184.2005. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. J Auton Nerv Syst 45: 139–147, 1993. doi: 10.1016/0165-1838(93)90125-E. [DOI] [PubMed] [Google Scholar]
- 26.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Mano T, Iwase S. Changes in muscle sympathetic nerve activity and calf blood flow during static handgrip exercise. Eur J Appl Physiol Occup Physiol 60: 277–281, 1990. doi: 10.1007/BF00379396. [DOI] [PubMed] [Google Scholar]
- 28.Salmanpour A, Brown LJ, Shoemaker JK. Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. J Neurosci Methods 193: 343–355, 2010. doi: 10.1016/j.jneumeth.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R, Shoemaker JK. Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. J Neurophysiol 105: 2830–2842, 2011. doi: 10.1152/jn.00814.2010. [DOI] [PubMed] [Google Scholar]
- 30.Salmanpour A, Frances MF, Goswami R, Shoemaker JK. Sympathetic neural recruitment patterns during the Valsalva maneuver. Conf Proc IEEE Eng Med Biol Soc 2011: 6951–6954, 2011. doi: 10.1109/IEMBS.2011.6091757. [DOI] [PubMed] [Google Scholar]
- 31.Scott DW. On optimal and data-based histograms. Biometrika 66: 605–610, 1979. doi: 10.1093/biomet/66.3.605. [DOI] [Google Scholar]
- 32.Seals DR, Chase PB, Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol (1985) 64: 2190–2196, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19: 313–349, 1991. doi: 10.1249/00003677-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker JK, Badrov MB, Al-Khazraji BK, Jackson DN. Neural control of vascular function in skeletal muscle. Compr Physiol 6: 303–329, 2015. doi: 10.1002/cphy.c150004. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 36.Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010. doi: 10.1113/jphysiol.2010.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tompkins RP, Melling CW, Wilson TD, Bates BD, Shoemaker JK. Arrangement of sympathetic fibers within the human common peroneal nerve: implications for microneurography. J Appl Physiol (1985) 115: 1553–1561, 2013. doi: 10.1152/japplphysiol.00273.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 39.Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res 76: 127–131, 1995. doi: 10.1161/01.RES.76.1.127. [DOI] [PubMed] [Google Scholar]
- 41.Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Williamson JW. The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95: 1043–1048, 2010. doi: 10.1113/expphysiol.2009.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]