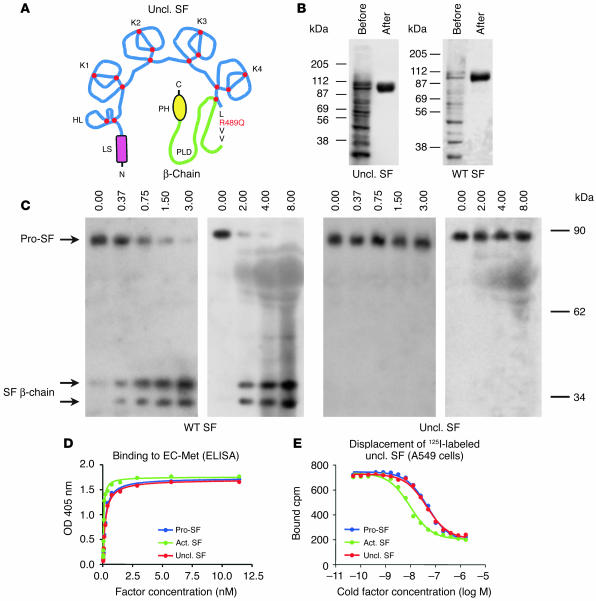
Figure 1.
Uncleavable SF is resistant to proteolytic activation, but binds Met with high affinity. (A) Schematic representation of uncleavable SF (Uncl. SF; from N to C terminus): LS, leader sequence; HL, hairpin loop; K1–K4, kringle 1–4; PLD, protease-like domain; PH, poly-His. The Arg-to-Gln substitution in the proteolytic site is shown in red. Red dots indicate disulfide bridges. (B) Purification of wild-type or uncleavable SF by affinity chromatography, before and after purification. Proteins were resolved by nonreducing SDS-PAGE. (C) Uncleavable SF is resistant to proteolytic conversion by uPA and FBS. Proteins were resolved by SDS-PAGE in reducing conditions and analyzed by Western blot using antibodies against the β-chain of SF. The 2 SF β-chain bands correspond to differently glycosylated forms. (D) Binding of uncleavable SF to purified extracellular Met (EC-Met) as assessed by ELISA. Bound SF was revealed using biotinylated anti-SF. (E) Binding of iodinated uncleavable SF to A549 human lung carcinoma cells. 125I-labeled uncleavable SF was displaced using increasing concentrations of cold pro-SF, active SF (Act. SF), or uncleavable SF.