Abstract
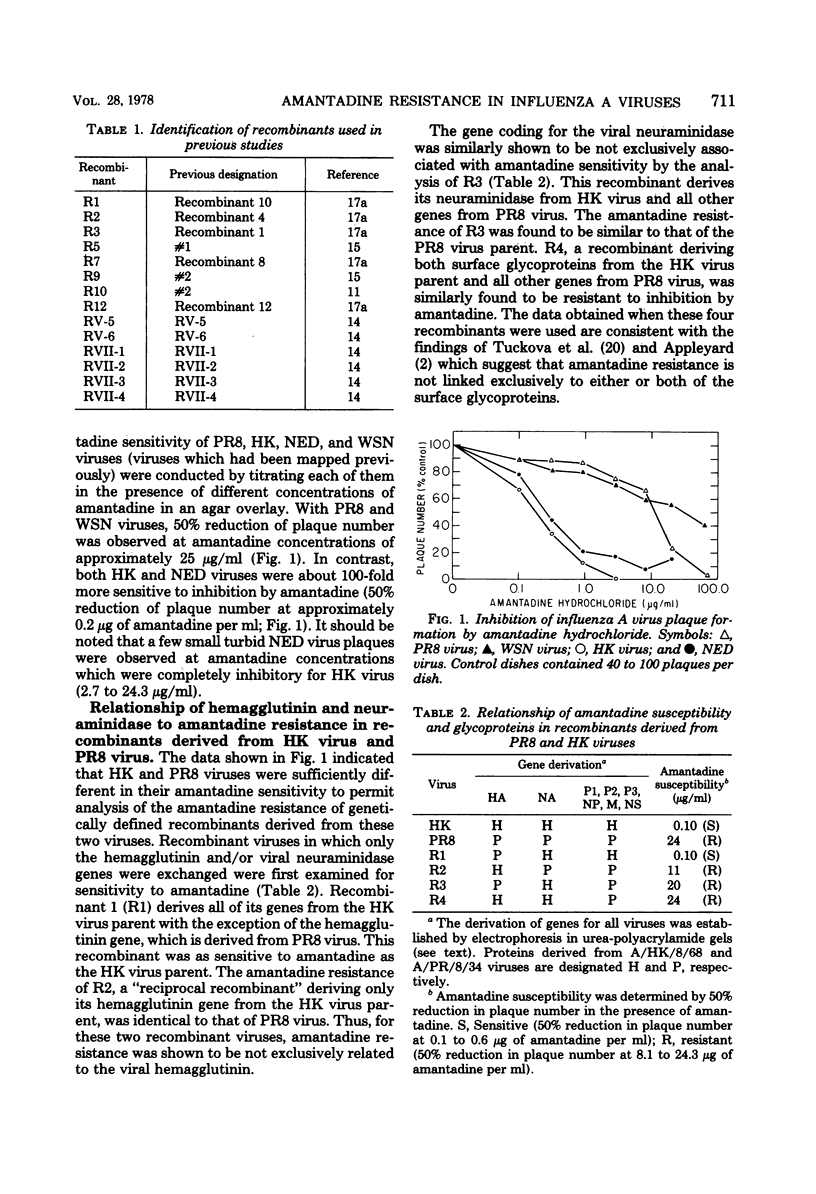
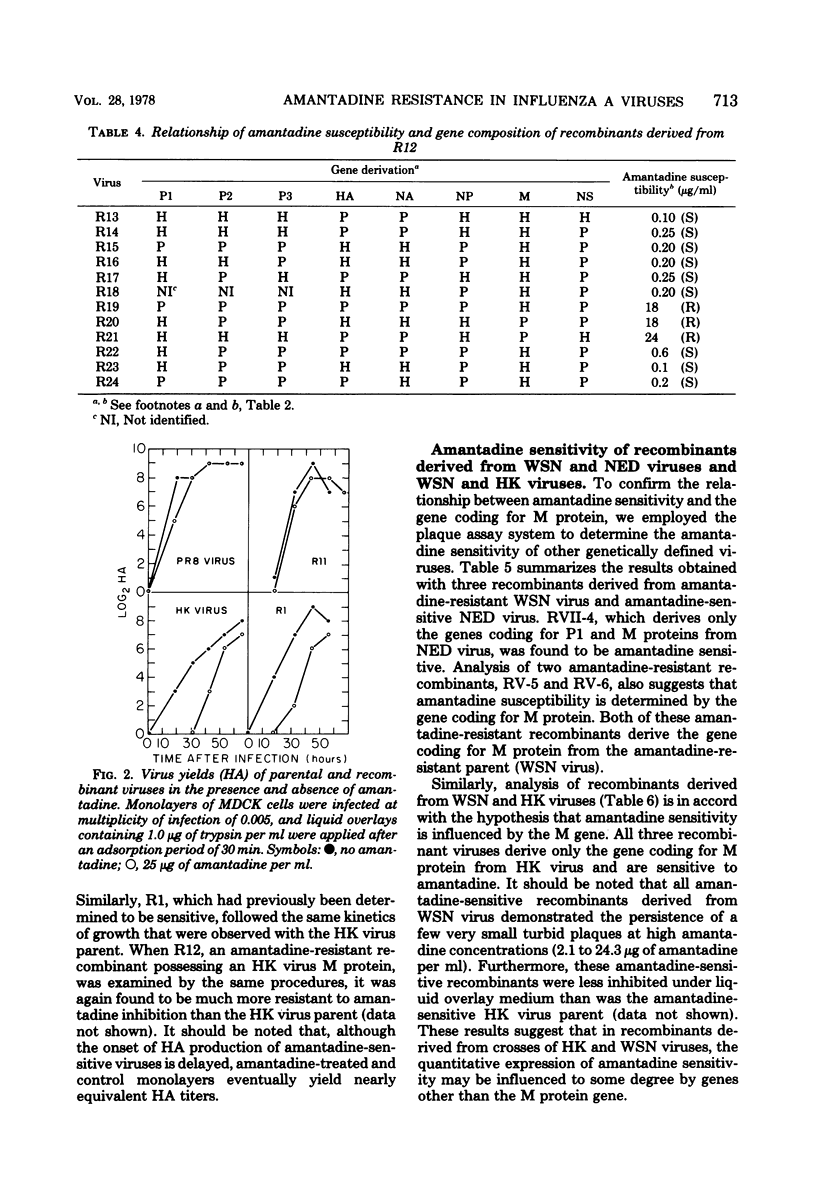
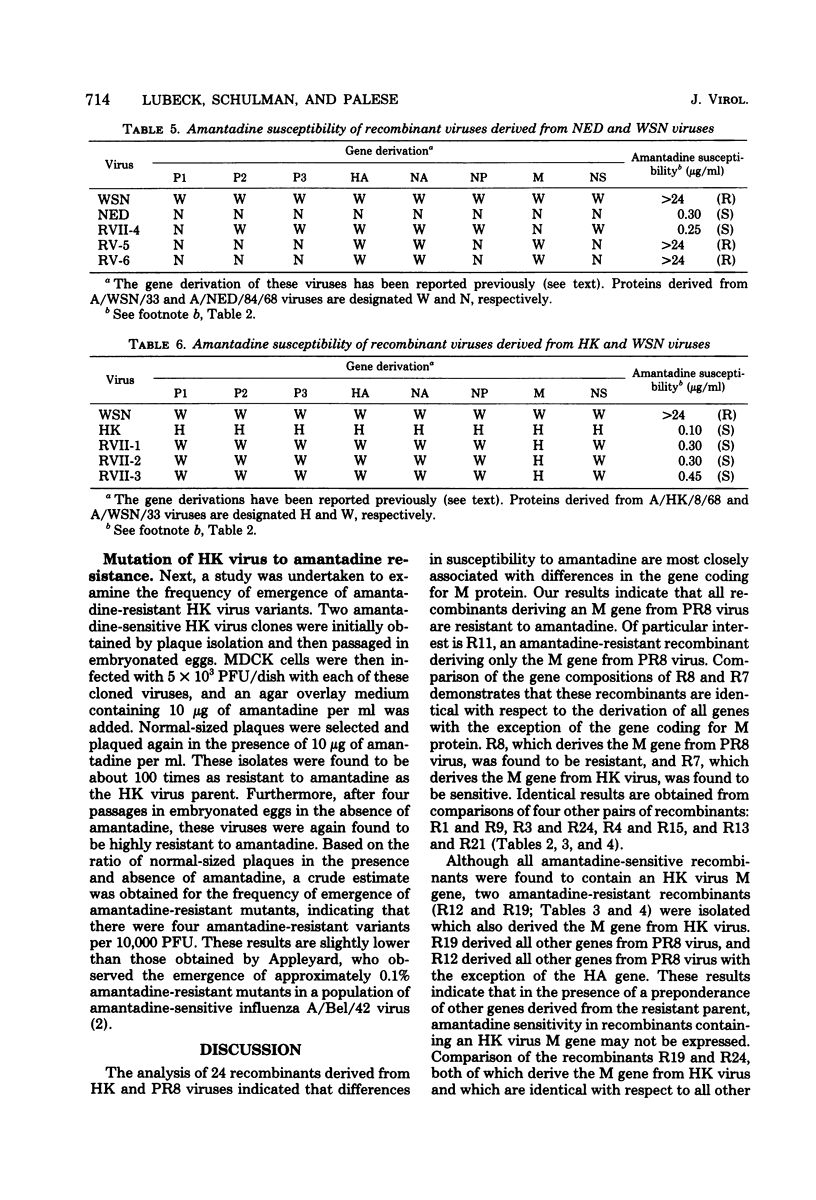
Influenza A virus recombinants derived from "resistant" and "sensitive" parental viruses were examined for susceptibility to inhibition by amantadine. Correlation of gene constellation and amantadine susceptibility revealed that the gene coding for M protein influences sensitivity or resistance to amantadine. All recombinants which derived an M protein from an amantadine-resistant parent were found to be resistant to amantadine. All amantadine-sensitive recombinants derived an M gene from the amantadine-sensitive parent. However, a few amantadine-resistant recombinants which derived an M gene from the sensitive parent were also isolated, suggesting that the expression of amantadine sensitivity in these recombinants may be influenced by other genes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W. A single gene determines the host range of influenza virus. Nature. 1977 Dec 15;270(5638):617–618. doi: 10.1038/270617a0. [DOI] [PubMed] [Google Scholar]
- Appleyard G. Amantadine-resistance as a genetic marker for influenza viruses. J Gen Virol. 1977 Aug;36(2):249–255. doi: 10.1099/0022-1317-36-2-249. [DOI] [PubMed] [Google Scholar]
- DAVIES W. L., GRUNERT R. R., HAFF R. F., MCGAHEN J. W., NEUMAYER E. M., PAULSHOCK M., WATTS J. C., WOOD T. R., HERMANN E. C., HOFFMANN C. E. ANTIVIRAL ACTIVITY OF 1-ADAMANTANAMINE (AMANTADINE). Science. 1964 May 15;144(3620):862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- GRUNERT R. R., MCGAHEN J. W., DAVIES W. L. THE IN VIVO ANTIVIRAL ACTIVITY OF 1-ADAMANTANAMINE (AMANTADINE). I. PROPHYLACTIC AND THERAPEUTIC ACTIVITY AGAINST INFLUENZA VIRUSES. Virology. 1965 Jun;26:262–269. doi: 10.1016/0042-6822(65)90273-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann C. E., Neumayer E. M., Haff R. F., Goldsby R. A. Mode of Action of the Antiviral Activity of Amantadine in Tissue Culture. J Bacteriol. 1965 Sep;90(3):623–628. doi: 10.1128/jb.90.3.623-628.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalninya V. A., Indulen M. K. Effect of adamantane derivatives on the activity of orthomyxovirus RNA-dependent RNA polymerase. Acta Virol. 1976 Aug;20(4):343–346. [PubMed] [Google Scholar]
- Kato N., Eggers H. J. Inhibition of uncoating of fowl plague virus by l-adamantanamine hydrochloride. Virology. 1969 Apr;37(4):632–641. doi: 10.1016/0042-6822(69)90281-5. [DOI] [PubMed] [Google Scholar]
- Long W. F., Olusanya J. Adamantanamine and early events following influenza virus infection. Arch Gesamte Virusforsch. 1972;36(1):18–22. doi: 10.1007/BF01250291. [DOI] [PubMed] [Google Scholar]
- NEUMAYER E. M., HAFF R. F., HOFFMAN C. E. ANTIVIRAL ACTIVITY OF AMANTADINE HYDROCHLORIDE IN TISSUE CULTURE AND IN OVO. Proc Soc Exp Biol Med. 1965 Jun;119:393–396. doi: 10.3181/00379727-119-30191. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. Mapping of the influenza virus genome. II. Identification of the P1, P2, and P3 genes. Virology. 1977 Jan;76(1):114–121. doi: 10.1016/0042-6822(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P. Identification of the defective genes in three mutant groups of influenza virus. J Virol. 1977 Mar;21(3):1196–1204. doi: 10.1128/jvi.21.3.1196-1204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rott R., Orlich M., Harms E., Rohde W. Correlation of pathogenicity and gene constellation of an influenza A virus (fowl plague). I. Exchange of a single gene. Virology. 1977 Aug;81(1):74–80. doi: 10.1016/0042-6822(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977 Oct;24(1):170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J., Armstrong J. A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978 Jan;38(1):97–110. doi: 10.1099/0022-1317-38-1-97. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]


