Abstract
Objective:
To evaluate the feasibility and efficacy of accelerated hypofractionated radiation with concomitant chemotherapy (AHFx-RT-CT) in locally advanced squamous cell carcinoma (SCC) of the lung.
Methods:
36 patients were enrolled in this study (CTRI/2013/11/004143). Patients in Arm A (n = 18) received neoadjuvant chemotherapy (NACT) (paclitaxel 200 mg m−2 and carboplatin area under the curve 5) followed by external radiotherapy (60 Gy/30 fractions/6 weeks). Patients in Arm B (n = 18) received NACT as in Arm A followed by AHFx-RT (48 Gy/20 fractions/4 weeks) with concomitant chemotherapy (cisplatin 30 mg m−2 weekly). Primary end points included comparative evaluation of overall locoregional response rates (ORRs) and progression-free survival (PFS). Secondary end points included toxicity, quality of life (QOL) and overall survival (OS).
Results:
The median follow-up duration was 15 months. The ORR at first follow-up (72.2% vs 44%, p = 0.06) and at 1 year after treatment completion (61% vs 5.5%, p = 0.04) were superior in Arm B. The median PFS (17 vs 5.36 months; p = 0.053) and OS (24.73 vs 12.33 months; p = 0.007) were also superior in Arm B. Grade ≥3 acute pharyngitis/oesophagitis was less in Arm B (p = 0.05). Improvement of emotional function, cognitive function and chest pain was observed in Arm B.
Conclusion:
The study suggests that AHFx-RT-CT is feasible for locally advanced SCC of the lung with improved response rate, survival, QOL and favourable toxicity.
Advances in knowledge:
To the best of our knowledge, this is the first study comparing conventionally fractionated radiation with AHFx-RT-CT. Addition of low-dose weekly cisplatin as radiosensitizer may be the potential factor responsible for improved response rate, survival and favourable toxicity in the study arm despite lower biological effective dose.
INTRODUCTION
Lung cancer comprises around 13% of the total estimated global burden of cancer; more than half of this entire burden is borne by the developing countries.1 Lung cancer ranks top in incidence as well as mortality among all cancers prevalent in Indian male population.2 About 86% of patients of lung cancer in India present with Stage III–IV disease, of which the locally advanced group accounts for 29% of overall patients. Nearly one-third of the patients are surgically unresectable.3 Current treatment guidelines recommend a combined multimodality approach of concurrent chemotherapy and radiotherapy for locally advanced non-small-cell lung cancer (LA-NSCLC).4–7 Nevertheless, concurrent chemoradiation is associated with high incidence of toxicity and poor compliance. There is no clear consensus on the chemotherapy regimen, radiation dose and optimal fractionation schedule.8 In addition, extensive disease burden, poor performance status of the patients and limited resources often preclude us from the practice of concurrent chemoradiation. Hence, sequential chemoradiation is often preferred in our practice. Till date, standard fractionation schedules of radiotherapy have resulted in dismal local control and survival rates.9 Accelerated repopulation of tumour cells during radiation therapy bears a strong correlation with poor local control and outcome. Radiation Therapy and Oncology Group (RTOG) trials for patients treated with concurrent chemoradiotherapy for Stage III non-small-cell lung cancer also demonstrated that prolonged treatment time leads to approximately 2% increase in the risk of death for each day of prolongation in therapy.10 Dose escalation beyond 60 Gy with standard fractionation has also failed to yield any encouraging result for patients with unresectable Stage III lung cancer.11 It is therefore imperative to achieve adequate biological effective dose (BED) at constant or reduced overall treatment duration to improve local control. A radiation dose of 55 Gy/20 fractions/4 weeks has been the preferred treatment in the UK for patients with inoperable non-small-cell lung cancer and good performance status.12 However, prospective randomized studies are still lacking to clarify about the ideal fractionation schedule, appropriate BED and chemotherapy regimen.
The present prospective randomized Phase II study evaluates the feasibility and efficacy of accelerated hypofractionated radiotherapy with concomitant chemotherapy in locally advanced squamous cell lung cancer at our centre.
METHODS AND MATERIALS
Patients
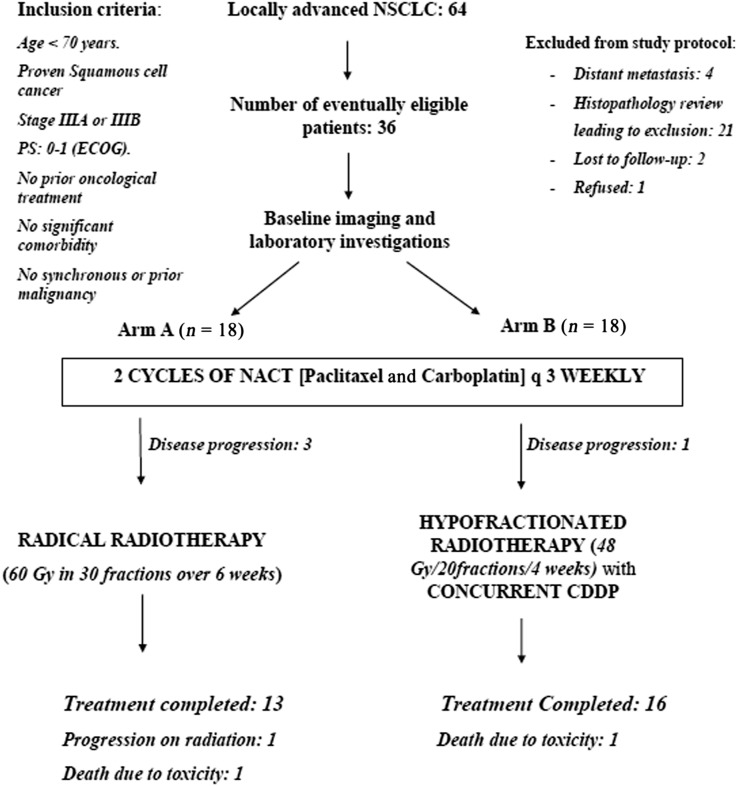
36 patients with locally advanced unresectable squamous cell carcinoma (SCC) of the lung were enrolled in a randomized controlled trial between October 2011 and July 2013. Eligibility criteria (Figure 1) included newly diagnosed patients (previously untreated) of biopsy-proven SCC of the lung with a performance status score of Eastern Co-operative Oncology Group 0–1, Stages IIIA and IIIB, without significant haematological or other systemic (renal, hepatic or pulmonary) impairments. Patients with hypersensitivity to platinum agents or comorbidities that can adversely affect treatment and outcome or those who had prior or synchronous malignancies were excluded from the study. Patient confidentiality was maintained by assigning identity code numbers. The protocol was approved by the institutional ethics committee (IESC/T-414). The trial was registered with the Clinical Trial Registry of India (registration number: CTRI/2013/11/004143). Written informed consent was obtained from all patients before the beginning of the treatment.
Figure 1.
Consort diagram of the study. CDDP, cisplatin; ECOG, Eastern Co-operative Oncology Group; 18F-FDG PET/CT, fluorine-18 fludeoxyglucose positron emission tomography/CT; NACT, neoadjuvant chemotherapy; NSCLC, non-small-cell lung cancer; PS, performance status.
Pre-treatment assessment
All patients underwent detailed clinical evaluation: complete blood count; liver and kidney function tests; contrast-enhanced CT (CECT) scans of the chest, abdomen and brain; pulmonary function test; and fluorine-18 fludeoxyglucose positron emission tomography/CT scan of the whole body. Adequate haematological and biochemical parameters (Supplementary document 1) were ensured before accrual.
Randomization
The study was designed as an open-label parallel Phase II randomized controlled trial. All patients fulfilling the inclusion criteria were randomly allocated at 1 : 1 ratio to the two treatment arms as per random numbers generated by the computer-generated randomization table.
Neoadjuvant chemotherapy
Two cycles of neoadjuvant chemotherapy were administered at an interval of 3 weeks. Paclitaxel (200 mg m−2 body surface area) and carboplatin (area under the curve 5) were administered in all patients (n = 36) as intravenous infusion on Day 1 of each cycle. The dose of chemotherapy drugs was reduced by 20% if the day-19 platelet count dropped <100,000 mm3 and was deferred if the day-19 platelet count was <70,000 mm3.
External beam radiation therapy
After 3–4 weeks of completion of neoadjuvant chemotherapy, eligible patients in the Arm A received external beam radiotherapy to a total dose of 60 Gy in 30 fractions over 6 weeks and patients in the study arm (Arm B) received 48 Gy in 20 fractions over 4 weeks along with concomitant cisplatin (30 mg m−2 body surface area once every week).
All patients were planned by the three-dimensional conformal radiation therapy technique. CT simulation was carried out with a Brilliance BigBore™ CT simulator (Phillips Medical System, Cleveland, OH) with fiducial markers. CECT (with oral and intravenous contrast) of the neck, thorax and upper abdomen was carried out using 3-mm slice thickness. The images were transferred to the Eclipse™ v. 6.5 (Varian Medical Systems, Palo Alto, CA) treatment-planning system. The definition of all volumes was in accordance with the 1993 International Commission on Radiation Units and Measurements report 50. Gross tumour volume was delineated encompassing the volume of pre-chemotherapy gross primary disease and the involved nodes. A 6-mm margin was given around the gross tumour volume to form the clinical target volume. The planning target volume (PTV) was defined as the clinical target volume enlarged by a margin of 10–15 mm, based on the extent of respiratory movement observed in the fluoroscopic simulator. The oesophagus, heart, spinal cord and normal lung were delineated as critical organs following the RTOG guidelines. Radiation was delivered by a Clinac®-2300 CD linear accelerator (Varian Medical Systems) using 6-MV photons. The treatment plan was approved when 95% of the PTV received at least 95% of the prescribed dose. The other criteria were the volume of PTV receiving 110% or more of the prescribed dose (V110%) should be <10% and the volume of PTV receiving 115% or more of the prescribed dose (V115%) of the PTV should be <1%. Dose limits used for the surrounding critical structures were the maximum dose (Dmax) and mean dose (Dmean) for the oesophagus (<60 Gy and <34 Gy), length of the oesophagus encompassed by 60 Gy <8 cm, Dmax for the spine <45 Gy, V45 for the spine <1 cm3, V20 for the normal lung (excluding the PTV) <30%, Dmean for the heart <26 Gy, V30 (percentage volume of heart receiving ≥30 Gy) for the heart <46% and V40 (percentage volume of heart receiving ≥40 Gy) for the heart <35%. The BED was estimated using the linear-quadratic model equation. The α/β ratio of 10 was used for tumour control probability and acute radiation-induced morbidity, whereas 3 was used for late normal tissue effects. In Arm B, the equivalent dose at 2 Gy/fraction (EQD2) was calculated for target volumes and critical organs.
Quality control
Two radiation oncologists of the Department of Radiation Oncology, All India Institute of Medical Sciences, reviewed and approved the radiotherapy records of each randomized patient including the time–dose fractionation schedule, radiation treatment planning, total tumour dose and dose to critical organs as per the protocol. The documentation of acute and late toxicities was also verified.
Evaluation during treatment
Patients were monitored during external beam radiotherapy with clinical examination and laboratory investigations for the assessment and grading of acute treatment-related morbidities. The Common Terminology Criteria for Adverse Events v. 3.0 were used for scoring of acute toxicity. Toxicities were managed conservatively.
Response assessment and follow-up
Patients were evaluated with CECT of the chest, brain and abdomen and fluorine-18 fludeoxyglucose positron emission tomography/CT of the whole body after 6 weeks of treatment completion. Subsequent follow-up visits were made every 6 weeks for the first year, every 3 months for the next 2 years and every 6 months thereafter. Complete response (CR), partial response, stable disease and progressive disease were defined as per the revised Response Evaluation Criteria in Solid Tumours criteria.13 Overall locoregional response rate (ORR) was defined as the sum of CR and partial response. Patients were simultaneously evaluated for any treatment-induced delayed morbidities using RTOG late morbidity scoring criteria.14
Quality of life
Quality of life (QOL) analysis was made using European Organisation for Research and Treatment of Cancer QOL questionnaire C30 and LC13. The questionnaire was answered by patients before the start of the treatment and at the time of first post-treatment evaluation. The raw scores and functional scores were calculated. Pre- and post-treatment scores for different items were calculated and the difference of value was determined for each item. Higher scores for symptoms and QOL metrics indicated greater severity of symptoms and better global QOL, respectively.
Statistical analysis
The primary end points of this Phase II pilot study included the evaluation and comparison of ORR at 6 weeks and 1 year after the completion of treatment, and comparative evaluation of progression-free survival (PFS) between the two arms. Secondary end points included assessment and comparison of toxicities, overall survival (OS) and QOL parameters between the two arms.
At the time of designing the study, there was no existing literature to guide in calculation of the sample size. Based on the available resources and logistics, a sample size of 60 patients was planned. However, because of reasons and constraints beyond control, we could enrol only 36 patients as per the inclusion criteria of the study.
The categorical clinicodemographic characteristics of the two treatments were compared using Fisher's exact test. For continuous variables, mean and median values were compared using the t-test. Fisher's exact test was applied to compare the ORRs between the two arms. PFS was defined as the period from the date of diagnosis to the date of locoregional failure, distant metastasis or last follow-up. OS was defined as the period from the date of diagnosis to death or last follow-up. PFS and OS were estimated using the Kaplan–Meier method. Log-rank test was used to compare the pattern of PFS and OS between the two arms. Differences in toxicity distributions were examined using Fisher's exact test. The two-sample Wilcoxon rank-sum test was used to compare the QOL parameters among the two arms. All reported p-values are two sided. A p-value of ≤0.05 was considered significant. The SPSS® v. 20.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used for all statistical analyses.
RESULT
Patient demographics
64 patients were initially staged as LA-NSCLC during the study period. 36 patients fulfilling the inclusion criteria were enrolled (Figure 1).
The median age of the study cohort was 56.4 years (range: 42–70 years). The male : female ratio was 17 : 1. The majority of them were smokers (n = 34) and had Stage IIIB (n = 21). Stage and other clinicodemographic variables were evenly distributed among the two arms (Table 1). Common symptoms among the study cohort were cough (n = 23), chest pain (n = 17), shortness of breath (n = 16) and fever (n = 9).
Table 1.
Showing the demographic characteristics in the study cohort
| Parameters | Arm A (n = 18) | Arm B (n = 18) | p-value |
|---|---|---|---|
| Age (years) | |||
| Median (range) |
60 (42–70) |
55 (42–70) |
0.84 |
| Mean ± SD | 58 ± 8.48 | 56 ± 8.08 | |
| Sex | |||
| Male | 17 | 17 | 0.99 |
| Female | 1 | 1 | |
| Smoking | |||
| Smoker | 17 | 17 | 0.99 |
| Non-smoker | 1 | 1 | |
| Stage group | |||
| IIIA | 7 | 8 | 0.99 |
| IIIB | 11 | 10 | |
SD, standard deviation.
Treatment details
29 patients completed the intended treatment course (Arm A: 13 patients; Arm B: 16 patients; p = 0.40). The causes of incomplete treatment included disease progression (n = 5) and death due to toxicity (n = 2) (Figure 1). The median doses of paclitaxel in the two arms were 245 and 260.8 mg, respectively. Carboplatin dose was uniform in both arms. The median PTVs in both the arms were 789.5 and 764.6 mm3, respectively. Median Dmax (maximum point dose) of the oesophagus was higher in Arm A than in Arm B. Median Dmax of the spine was marginally higher in Arm B (Table 2). V30 and V40 of the heart were higher in Arm B (Table 2). The median number of concurrent chemotherapy cycles was 4.5 (range: 4–5) in Arm B. The median dose of concurrent weekly cisplatin was 45 mg (range: 40–56 mg). The mean overall treatment duration in Arm B was 12.07 ± 1.14 weeks and in Arm A was 14.78 ± 3.13 weeks (p = 0.03).
Table 2.
Showing the comparison of radiotherapy (RT) parameters between the two arms
| RT parameters | Arm A | Arm B |
|---|---|---|
| Radical | 13 | 16 |
| Dose (Gy)/fraction/time (weeks) | 60 Gy/30 fractions/6 weeks | 48 Gy/20 fractions/4 weeks |
| Median number of fields (range) | 3 (2–7) | 3 (2–4) |
| Median PTV (cm3) | 789.5 | 764.6 |
| Median heart V40 (%) (range) | 5.74 (0–41.36) | 18.44 (0–46.5) |
| Median heart V30 (%) (range) | 11 (0–45.79) | 23 (0–50.76) |
| Median spine Dmax (range) | 44.64 (35.15–50.09) | 48 (1.35–53.01) |
| Median oesophagus Dmax (range) | 63 (2.37–65) | 53 (48–55.13) |
| Median oesophagus Dmean (range) | 29.4 (2–50) | 25.41 (15–46.5) |
| Median (lung PTV) V20 (range) | 29.42 (5.56–63.74) | 21.44 (9.10–41.00) |
Dmax, maximum dose; Dmean, mean dose; PTV, planning target volume; V20, volume receiving ≥20 Gy; V30, volume receiving ≥30 Gy; V40, volume receiving ≥40 Gy.
Overall response to treatment
The ORR at 6 weeks after the completion of treatment was superior in Arm B (72.2%; n = 13) than in Arm A (44%; n = 8) (p = 0.06). CR was seen in 11.11% of patients in Arm B (n = 2), whereas none of the patients had CR in Arm A. At 12 months after the completion of treatment, the ORR was 61% in Arm B (n = 11) and 5.5% in Arm A (n = 1) (p = 0.04).
Toxicity
Acute toxicities
Two patients in Arm A and one patient in Arm B developed grade ≥3 haematological toxicities during treatment. The common grade ≥3 haematological toxicities were neutropaenia (one patient in each arm) followed by thrombocytopenia (one patient in Arm A) (Table 3). Eight patients in Arm A and four patients in Arm B (study arm) developed grade ≥3 non-haematological toxicities. The most common grade ≥3 non-haematological toxicities were peripheral neuropathy (Arm A: two patients, Arm B: two patients) and pharyngitis/oesophagitis (Arm A: three patients, Arm B: one patient; p = 0.05). Four patients required hospitalization (Arm A = three patients, Arm B = one patient). There were two grade 5 toxicities, one in each arm (hyponatraemia in Arm A and neutropaenia in Arm B). The rest of the acute toxicities were grade <3 and were comparable between the two arms. However, grades 1 and 2 skin toxicity were significantly higher in Arm A (p = 0.04).
Table 3.
Showing the comparative evaluation of acute toxicities in the two arms
| Toxicity | Subtypes | Grade | Arm A | Arm B | p-value |
|---|---|---|---|---|---|
| Haematological | Anaemia | Grade 1/2 | 0 | 1 | 0.99 |
| Grade ≥3 | 0 | 0 | – | ||
| Neutropaenia | Grade 1/2 | 0 | 1 | 0.99 | |
| Grade ≥3 | 1 | 1 | 0.99 | ||
| Thrombocytopenia | Grade 1/2 | 1 | 2 | 0.87 | |
| Grade ≥3 | 1 | 0 | 0.99 | ||
| Non-haematological | Skin reactions | Grade 1/2 | 6 | 1 | 0.04 |
| Grade ≥3 | 1 | 0 | 0.99 | ||
| Anorexia | Grade 1/2 | 7 | 9 | 0.63 | |
| Grade ≥3 | 0 | 1 | 0.99 | ||
| Mucositis | Grade 1/2 | 4 | 1 | 0.07 | |
| Grade ≥3 | 0 | 0 | – | ||
| Laryngitis | Grade 1/2 | 3 | 2 | 0.99 | |
| Grade ≥3 | 0 | 0 | – | ||
| Pharyngitis/oesophagitis | Grade 1/2 | 7 | 10 | 0.83 | |
| Grade ≥3 | 3 | 1 | 0.05 | ||
| Pneumonitis | Grade 1/2 | 6 | 3 | 0.26 | |
| Grade ≥3 | 1 | 0 | 0.99 | ||
| Peripheral neuropathy | Grade 1/2 | 4 | 4 | 0.99 | |
| Grade ≥3 | 2 | 2 | 0.99 | ||
| Hyponatraemia | Grade 1/2 | 0 | 0 | – | |
| Grade ≥3 | 1 | 0 | 0.99 |
Late toxicities
At the last follow-up, 14 patients (7 patients in each arm) developed late toxicities, all of which were of grade <3. Lung fibrosis was observed in five patients (Arm A two patients, Arm B three patients); late grades 1 and 2 oesophageal morbidity occurred in two patients in each arm. Three patients in Arm A developed late grade 1 skin morbidity. One patient in each arm developed grade 1 neurological toxicity. Comparison of late toxicities between the two arms did not confer any statistical significance.
Survival
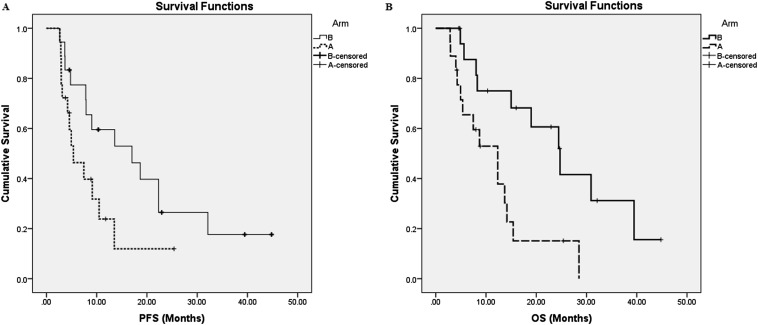
The median follow-up duration was 15 months (range: 2.9–46.8 months). The median PFS in Arm B was 17 months, whereas in Arm A, it was 5.36 months (p = 0.053, log-rank test) (Figure 2a). The median OS in Arms B and A were 24.73 and 12.33 months, respectively (p = 0.007, log-rank test) (Figure 2b). The rates of OS at 1 year were 52% and 75% in Arms A and B, respectively.
Figure 2.
Kaplan–Meier survival curves for progression-free survival (PFS) (a) and overall survival (OS) (b) of the two arms.
Quality of life
At baseline, all patients answered the European Organisation for Research and Treatment of Cancer quality of life questionnaire C30 and LC13. Baseline functioning and symptom scores were well balanced between the two arms. At first follow-up after treatment completion, 28 patients (completing the protocol-specified treatment) answered the same questionnaire. Post-treatment emotional functioning was significantly better in Arm B (p = 0.009) (Table 4). A similar improvement was also observed in cognitive functioning in patients of Arm B at the time of first post-treatment assessment (p = 0.04). A borderline improvement in social functioning was also noted in patients of Arm B (p = 0.07). According to the LC13, alopecia showed a worsening trend in patients of Arm B (p = 0.09) at first follow-up; also, patients in Arm B achieved a significant post-treatment improvement of chest pain (p = 0.04). No other significant difference in QOL parameters was observed between the two treatment arms.
Table 4.
Comparative evaluation of baseline (pre-T/t) and post treatment (post-T/t) quality of life parameters [according to the European Organisation for Research and Treatment of Cancer quality of life questionnaire (QLQ) C30 and LC13]
| Parameters | Arm A, median (range) | Arm B, median (range) | p-value |
|---|---|---|---|
| Global health status | |||
| Pre-T/t | 41.67 (0–58.33) | 50 (8.33–66.67) | 0.24 |
| Post-T/t | 58.33 (8.33–100) | 66.67 (41.67–100) | 0.44 |
| Physical functioning | |||
| Pre-T/t | 53.33 (6.67–100) | 73.33 (13.33–100) | 0.13 |
| Post-T/t | 73.33 (0–80) | 80 (53.33–100) | 0.14 |
| Role functioning | |||
| Pre-T/t | 50 (0–100) | 83.33 (0–100) | 0.12 |
| Post-T/t | 83.33 (0–100) | 100 (66.67–100) | 0.25 |
| Emotional functioning | |||
| Pre-T/t | 53.33 (16.67–100) | 41.67 (8.33–100) | 0.81 |
| Post-T/t | 66.67 (16.67–100) | 91.66 (41.66–100) | 0.009 |
| Cognitive functioning | |||
| Pre-T/t | 66.67 (16.67–100) | 83.33 (16.67–100) | 0.41 |
| Post-T/t | 75 (33.33–100) | 100 (50–100) | 0.04 |
| Social functioning | |||
| Pre-T/t | 66.67 (0–100) | 83.33 (0–100) | 0.93 |
| Post-T/t | 66.67 (0–100) | 100 (33.33–100) | 0.07 |
| Fatigue | |||
| Pre-T/t | 66.67 (0–100) | 44.33 (0–100) | 0.21 |
| Post-T/t | 33.33 (11–88.9) | 22.33 (0–100) | 0.37 |
| Nausea and vomiting | |||
| Pre-T/t | 0 (0–66.67) | 16.67 (0–66.67) | 0.41 |
| Post-T/t | 0 (0–66.67) | 0 (0–33.33) | 0.84 |
| Pain | |||
| Pre-T/t | 50 (0–100) | 33.33 (0–100) | 0.52 |
| Post-T/t | 33.33 (0–66.67) | 33.33 (0–83.33) | 0.19 |
| Dyspnoea according to QLQ C30 | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (0–100) | 0.19 |
| Post-T/t | 16.67 (0–100) | 33.33 (0–33.33) | 0.83 |
| Insomnia | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (0–66.67) | 0.49 |
| Post-T/t | 0 (0–66.67) | 33.33 (0–66.67) | 0.77 |
| Anorexia | |||
| Pre-T/t | 66.67 (0–100) | 66.67 (0–100) | 0.99 |
| Post-T/t | 33.33 (0–100) | 33.33 (0–100) | 0.63 |
| Constipation | |||
| Pre-T/t | 0 (0–33.33) | 0 (0–66.67) | 0.99 |
| Post-T/t | 0 (0–66.67) | 0 (0–66.67) | 0.44 |
| Diarrhoea | |||
| Pre-T/t | 0 (0–33.33) | 0 (0–33.33) | 0.83 |
| Post-T/t | 0 | 0 | 0.99 |
| Financial difficulty | |||
| Pre-T/t | 66.67 (0–100) | 66.67 (0–100) | 0.66 |
| Post-T/t | 66.67 (0–100) | 33.33 (0–66.67) | 0.23 |
| Dyspnoea according to LC13 | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (0–88.9) | 0.44 |
| Post-T/t | 33.33 (11.1–100) | 22.23 (0–55.67) | 0.29 |
| Cough according to LC13 | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (33.33–100) | 0.60 |
| Post-T/t | 33.33 (0–66.67) | 33.33 (0–66.67) | 0.62 |
| Haemoptysis according to LC13 | |||
| Pre-T/t | 0 (0–100) | 0 (0–100) | 0.87 |
| Post-T/t | 0 (0–33.33) | 0 (0–33.33) | 0.88 |
| Soreness of mouth according to LC13 | |||
| Pre-T/t | 0 (0–66.67) | 0 (0–66.67) | 0.27 |
| Post-T/t | 0 (0–33.33) | 0 (0–33.33) | 0.67 |
| Dysphagia according to LC13 | |||
| Pre-T/t | 0 (0–66.67) | 0 (0–100) | 0.75 |
| Post-T/t | 0 (0–66.67) | 0 (0–33.33) | 0.48 |
| Peripheral neuropathy according to LC13 | |||
| Pre-T/t | 0 (0–66.67) | 0 (0–66.67) | 0.99 |
| Post-T/t | 66.67 (0–66.67) | 66.67 (0–100) | 0.86 |
| Alopecia according to LC13 | |||
| Pre-T/t | 0 | 0 | 0.99 |
| Post-T/t | 33.33 (0–66.67) | 66.67 (0–100) | 0.09 |
| Pain chest according to LC13 | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (0–100) | 0.82 |
| Post-T/t | 66.67 (0–100) | 0 (0–66.67) | 0.04 |
| Pain in arm according to LC13 | |||
| Pre-T/t | 33.33 (0–66.67) | 33.33 (0–100) | 0.32 |
| Post-T/t | 16.67 (0–66.67) | 0 (0–66.67) | 0.52 |
| Pain in other parts according to LC13 | |||
| Pre-T/t | 33.33 (0–100) | 33.33 (0–100) | 0.62 |
| Post-T/t | 0 (0–66.67) | 0 (0–66.67) | 0.23 |
Bold values indicate significance on statistical analyses.
DISCUSSION
Hypofractionated radiotherapy with higher dose (>2.5 Gy) per fraction in LA-NSCLC has been explored in the past and has failed to show any benefit in terms of survival.15,16 Although the survival outcomes were unsatisfactory, the observations suggested possible merits of hypofractionated radiotherapy compared with conventional radiotherapy. The addition of concurrent chemotherapy may have resulted in improvement of outcome.
The present study, to the best of our knowledge, is the first reported randomized study comparing conventional fractionated radiation with accelerated hypofractionated radiation schedule and concurrent chemotherapy. The overall treatment concordance was numerically superior in Arm B. Similarly, the ORR and PFS were also marginally superior in Arm B. Furthermore, the study arm observed improvement in OS and some of the post-treatment QOL metrics. The lower incidence of grade ≥3 acute oesophagitis in the study arm could also be attributed to lower cumulative BED and relatively lower median oesophageal Dmax and Dmean.
A large number of studies including a meta-analysis have established the superiority of concurrent chemoradiation over sequential chemoradiation therapy.4–7 Thus, the differences in ORR, PFS and OS in Arm B may be ascribed to the concomitant chemoradiation schedule. However, in the present study, Arm B (study arm) received comparatively lower cumulative BED and EQD2 (59.5 and 49.6 Gy) than the sequential arm (72 and 60 Gy). Therefore, concomitant low-dose weekly cisplatin was added as a potential radiosensitizer to make the radiation dose equivalent in the two arms. Chemotherapy increases BED by approximately 8.8 Gy in the standard and modified fractionated radiotherapy in advanced head and neck cancers.17 A similar benefit of concomitant cisplatin cannot be ruled out in our study. The abbreviated course of radiation also nullifies any negative influence of accelerated repopulation of the tumour cells over locoregional response and survival. This may be reflected in the superior survival outcome in Arm B of our study in comparison with the previous studies.15,16 Although the demographic parameters were equivalent in the two arms, a relatively younger population in Arm B, as evidently seen by the numerical difference of median age, superior compliance and also good performance status may be the other putative reasons for the superior outcome in this arm.
In the present study, the heart dose–volume parameters were higher in the study arm but within the permissible limits. V30 of the heart were 11% and 23% in Arms A and B, respectively. V40 of the heart in Arms A and B were 5.74% and 18.44%, respectively. However, compared with the post hoc analysis of RTOG 0617, the higher heart dose did not translate into inferior survival in our study.18
A recent Phase II randomized trial (SOCCAR trial) compared sequential and concurrent chemotherapy with hypofractionated radiotherapy. The 2-year survival rates were 50% vs 46% for concurrent and sequential arm, respectively.19 The observations are in agreement with the findings in our report. However, the cumulative BED of the concurrent chemoradiation arms of the two studies were different (70 vs 59.5 Gy), and the relatively favourable grade ≥3 toxicity profile of the present study can be attributed to the less cumulative BED in the Arm B of our study. The rates of grade ≥3 oesophagitis and pneumonitis were 8.8% and 8.5%, respectively, in the concurrent chemoradiation arm of the SOCCAR trial, which is considerably higher than the findings of our report. Our study revealed acute grade ≥3 oesophagitis rate of 5% and no pneumonitis with concomitant chemoradiotherapy. The present study highlights the comparable survival outcome with lower acute morbidities.
Zhu et al20 (n = 34) reported similar rates of pulmonary toxicity despite delivering higher dose per fraction compared with our study (50 Gy at 2.5 Gy/fraction vs 48 Gy at 2.4 Gy/fraction). However, the survival outcome is superior in the present report. The presumed reason may be the use of concomitant chemotherapy in Arm B of our study.
Liu et al21 used a further higher accelerated dose fractionation schedule (60 Gy/20 fractions/4 weeks) with concomitant chemotherapy. The dose was further escalated to 75 Gy in a selected group of patients, although the treatment-related morbidity was prohibitively high without any significant advantage in ORR and survival (median PFS and OS of 10 and 13 months). The findings suggest that addition of concurrent chemotherapy along with injudicious escalation of BED can be hazardous and therefore merits a prudent approach to strike a balance between the survival outcome and toxicity.
The recent systemic review by Kaster et al22 demonstrated survival and toxicity outcomes associated with hypofractionated radiotherapy in LA-NSCLC. The included studies had the limitation of extreme heterogeneity with wide variations in dose prescriptions, survival and toxicities. However, a moderate linear relationship between BED and OS was established. For every 1-Gy increase in BED, the authors estimated an absolute OS benefit of 0.36–0.7%. Varying fractionation schedules, heterogeneous study population and different chemotherapy regimens preclude us from a comparative analysis of these studies. Nevertheless, all of these studies, including the present one, highlight the importance of selecting a radiation schedule to balance disease outcome and morbidity in the era of concomitant chemoradiation.
Although the cumulative BED in the study arm of the present report is less than that used in the above studies,12,19–21 the response rates, toxicity and survival outcomes were consistent with reported literature. The consistency in the results among the studies underscores the fact that treatment acceleration is as important as achieving a higher BED and EQD2. Moreover, the present regimen, although milder in comparison with some of the other contemporary series, is deemed suitable for our patients who had poor performance status, with compromised nutrition and heavy disease burden.23 The choice of single-agent cisplatin was based on earlier studies where the toxicity profile was more favourable with low-dose weekly cisplatin schedules.24 The study schedule was preffered owing to its shorter overall treatment duration, improved compliance and lesser morbidity with fewer hospitalizations required during treatment. It reduced the waiting time for radiotherapy in the machines in a high-volume cancer centre and therefore had a superior cost–benefit ratio compared with conventional radiation schedules.
The present study has the limitation of small sample size and limited follow-up. The OS in Arm B was close to survival outcomes described in recent studies of radiochemotherapy for LA-NSCLC. By contrast, the OS was marginally inferior in Arm A when compared with that of other studies.7 The possible explanation could be administration of fewer cycles of chemotherapy in this arm. Hence, the result must be interpreted with caution and obviates the need for further prospective studies with a larger sample size for validation of the superiority of abbreviated hypofractionated radiation schedule along with concomitant chemotherapy.
CONCLUSION
Hypofractionated accelerated radiotherapy with concomitant chemotherapy is a feasible option for management of locally advanced SCC of the lung. The present hypofractionated accelerated chemoradiation regimen reduces the overall treatment time and leads to superior compliance, response rate, survival and QOL with a lower cumulative BED. The acute toxicity was also limited in the study arm. Hypofractionated acclerated radiotherapy with concomitant chemotherapy reduces the waiting time for radiotherapy in the machines in a high-volume cancer centre. The study regimen, therefore, is also advantageous from the aspect of overall cost–benefit ratio. However, given the limitations of the small sample size and limited follow-up, the results need to be interpreted with caution. Further prospective studies with an adequate sample size and longer follow-up may be worthwhile to establish the superiority of accelerated hypofractionated radiation with concurrent chemotherapy.
Acknowledgments
ACKNOWLEDGMENTS
We acknowledge the inputs and contributions of Ms Arushi Vemprala in rectifying the grammatical and stylistic errors in use of English.
Contributor Information
Soumyajit Roy, Email: soumyajitroy8@gmail.com.
Sushmita Pathy, Email: drspathy@gmail.com.
Bidhu K Mohanti, Email: drbkmohanit@rediffmail.com.
Vinod Raina, Email: Vinodraina@hotmail.com.
Anand Jaiswal, Email: anand_jaiswal43@hotmail.com.
Rakesh Kumar, Email: rkphulia@yahoo.com.
Mani Kalaivani, Email: manikalaivani@yahoo.co.in.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Unchanging clinico-epidemiological profile of lung cancer in north India over three decades. Cancer Epidemiol 2010; 34: 101–4. doi: 10.1016/j.canep.2009.12.015 [DOI] [PubMed] [Google Scholar]
- 3.Malik PS, Sharma MC, Mohanti BK, Shukla NK, Deo S, Mohan A, et al. Clinico-pathological profile of lung cancer at AIIMS: a changing paradigm in India. Asian Pac J Cancer Prev 2013; 14: 489–94. doi: 10.7314/APJCP.2013.14.1.489 [DOI] [PubMed] [Google Scholar]
- 4.Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 2005; 23: 5910–17. doi: 10.1200/JCO.2005.03.070 [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–60. doi: 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999; 17: 2692–9. [DOI] [PubMed] [Google Scholar]
- 7.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 2181–90. doi: 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 8.O'Rourke N, Macbeth F. Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence. Clin Oncol (R Coll Radiol) 2010; 22: 347–55. doi: 10.1016/j.clon.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63: 324–33. doi: 10.1016/j.ijrobp.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 10.Kong FM, Zhao J, Wang J, Faivre-Finn C. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis 2014; 6: 336–47. doi: 10.3978/j.issn.2072-1439.2014.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshy M, Malik R, Sher DJ, Spiotto M, Mahmood U, Aydogan B, et al. The effect of radiotherapy dose on survival in stage III non-small-cell lung cancer patients undergoing definitive chemoradiotherapy. Clin Lung Cancer 2014; 15: 365–71. doi: 10.1016/j.cllc.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Prewett SL, Aslam S, Williams MV, Gilligan D. The management of lung cancer: a UK survey of oncologists. Clin Oncol (R Coll Radiol) 2012; 24: 402–9. doi: 10.1016/j.clon.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 15.Osti MF, Agolli L, Valeriani M, Falco T, Bracci S, De Sanctis V, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 85: e157–63. doi: 10.1016/j.ijrobp.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 16.Kepka L, Tyc-Szczepaniak D, Bujko K. Dose-per-fraction escalation of accelerated hypofractionated three-dimensional conformal radiotherapy in locally advanced non-small cell lung cancer. J Thorac Oncol 2009; 4: 853–61. doi: 10.1097/JTO.0b013e3181a97dda [DOI] [PubMed] [Google Scholar]
- 17.Fowler JF. Correction to Kasibhatla et al. How much radiation is the chemotherapy worth in advanced head and neck cancer? (Int J Radiat Oncol Biol Phys 2007; 68: 1491–1495). Int J Radiat Oncol Biol Phys 2008; 71: 326–9. [DOI] [PubMed] [Google Scholar]
- 18.Christodoulou M, Bayman N, McCloskey P, Rowbottom C, Faivre-Finn C. New radiotherapy approaches in locally advanced non-small cell lung cancer. Eur J Cancer 2014; 50: 525–34. doi: 10.1016/j.ejca.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 19.Maguire J, Khan I, McMenemin R, O'Rourke N, McNee S, Kelly V, et al. SOCCAR: a randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. Eur J Cancer 2014; 50: 2939–49. doi: 10.1016/j.ejca.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 20.Zhu ZF, Fan M, Wu KL, Zhao KL, Yang HJ, Chen GY, et al. A phase II trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol 2011; 98: 304–8. doi: 10.1016/j.radonc.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 21.Liu YE, Lin Q, Meng FJ, Chen XJ, Ren XC, Cao B, et al. High-dose accelerated hypofractionated three-dimensional conformal radiotherapy (at 3 Gy/fraction) with concurrent vinorelbine and carboplatin chemotherapy in locally advanced non-small-cell lung cancer: a feasibility study. Radiat Oncol 2013; 8: 198. doi: 10.1186/1748-717X-8-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaster TS, Yaremko B, Palma DA, Rodrigues GB. Radical-intent hypofractionated radiotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer 2015; 16: 71–9. doi: 10.1016/j.cllc.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Mohan A, Singh P, Kumar S, Mohan C, Pathak AK, Pandey RM, et al. Effect of change in symptoms, respiratory status, nutritional profile and quality of life on response to treatment for advanced non-small cell lung cancer. Asian Pac J Cancer Prev 2008; 9: 557–62. [PubMed] [Google Scholar]
- 24.Koning CC, Wouterse SJ, Daams JG, Uitterhoeve LL, van den Heuvel MM, Belderbos JS. Toxicity of concurrent radiochemotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer 2013; 14: 481–7. doi: 10.1016/j.cllc.2013.03.002 [DOI] [PubMed] [Google Scholar]