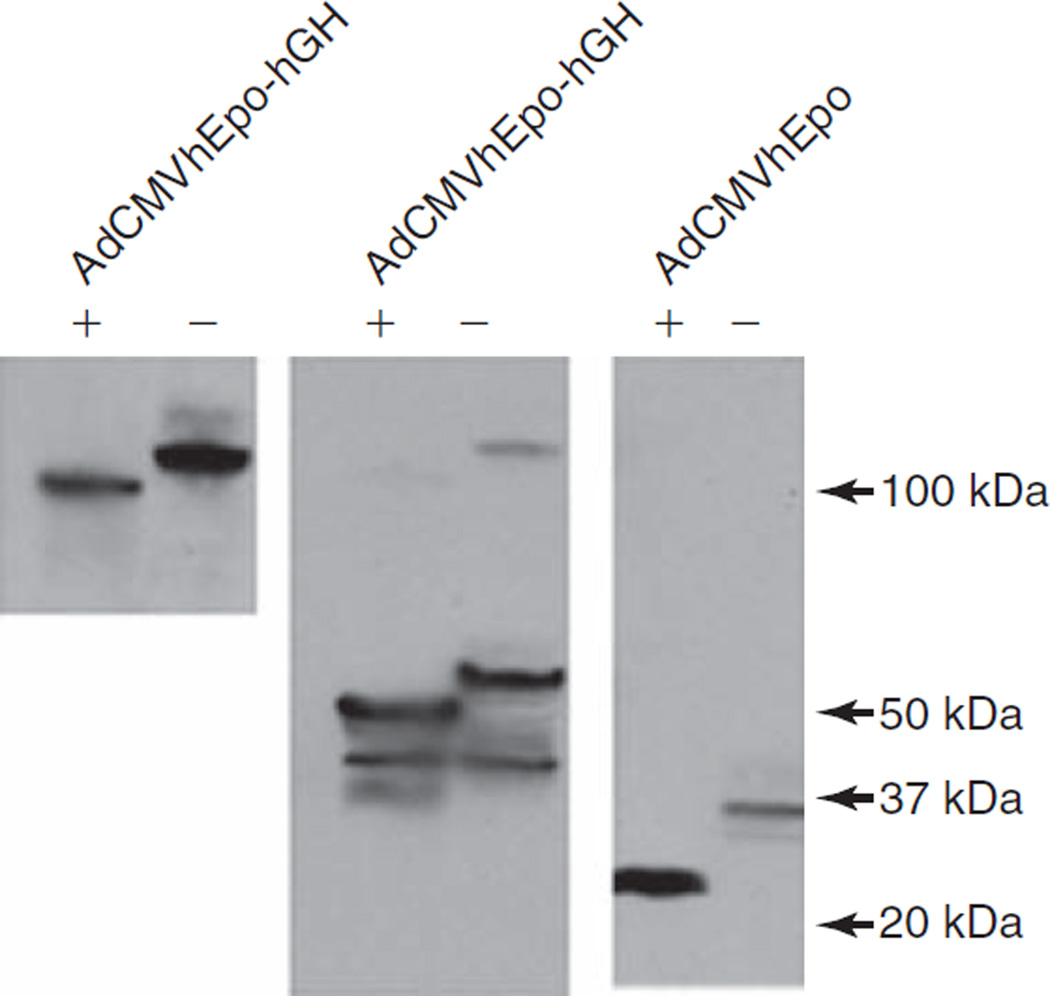
FIG. 2.
Western blot analyses of the glycosylation state of hEpo and hEpo–hGH protein. Forty-eight hours after transduction with AdCMVhEpo or AdCMVhEpo-hGH lysates were collected from transduced HEK 293 cells and either treated with N-glycosidase F (+) or left untreated (−). Proteins were then separated by gel electrophoresis, blotted, and reacted with anti-hEpo antibody. On the basis of the shift in electrophoretic mobility after incubation with N-glycosidase F, hEpo, the fusion protein hEpo–hGH, and the likely dimer of hEpo–hGH all were present in their glycosylated form with apparent molecular masses of ~34, ~56, and ~110 kDa, respectively. See Materials and Methods for additional details.