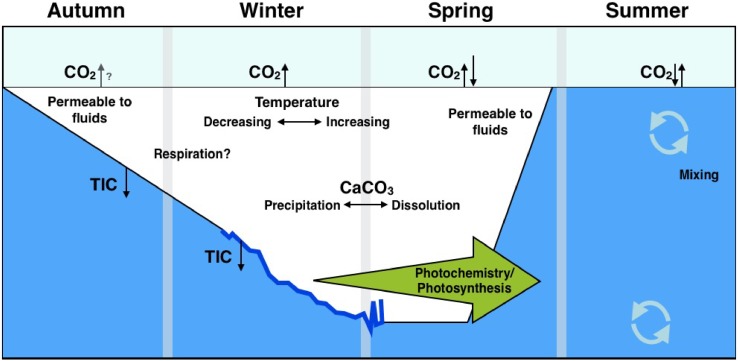
Fig. 3.
Summary of the various carbon cycling processes in the ocean related to sea ice. In autumn, carbon is rejected together with brine during sea ice formation, which sinks because of its high density (TIC total inorganic carbon). The permeability of the ice is determined by temperature, and the ice–air exchange of CO2 is governed by the difference in partial pressure of CO2 (pCO2sw) with the atmosphere. When sea ice melts, ikaite crystals within the ice dissolve and alter the alkalinity of surface waters, lowering pCO2sw, and stimulating uptake. Furthermore, if the ice is thin enough, sunlight can penetrate and stimulate photosynthesis. In areas without sea ice, the exchange with the atmosphere is determined by the pCO2sw difference between the air and the ocean surface.
Adapted from Miller et al. (2011)