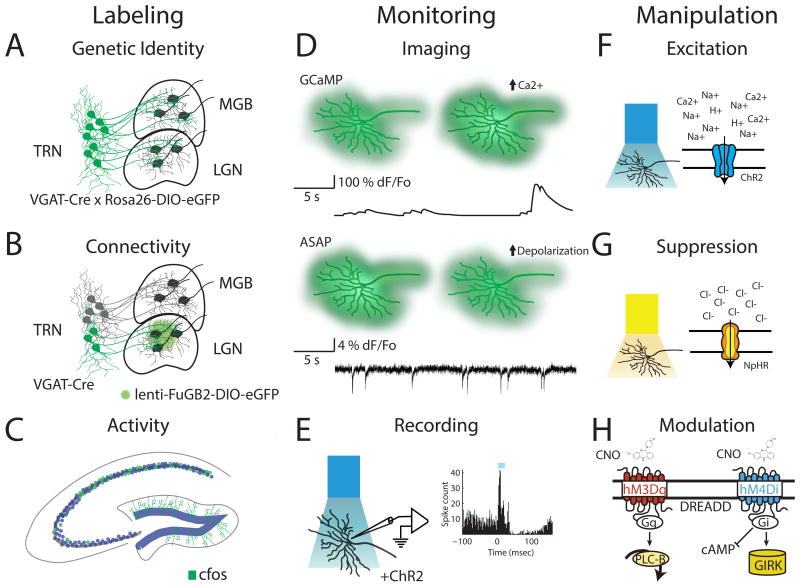
Figure 1. Tools for functional dissection of intact circuits in mice.
Schematic diagram illustrating available approaches to study circuits in the mouse include labeling, monitoring and manipulation. Labeling: Neuronal subpopulations can be marked based on their genetic identity (A), connectivity or both (B). In this example, inhibitory neurons in the thalamic reticular nucleus (TRN) are labelled either based on their expression of VGAT in a cre- reporter line alone (A) or also based on their projections identified using a retrograde lentiviral vector (B). In addition, elevated activity can be determined based on immediate early genes expression (e.g. cfos, panel C). Monitoring: Activity can be monitored in genetically defined populations through (D) genetically encoded fluorescent calcium (e.g. GCaMP) and voltage (e.g. ASAP) sensors (top panel) or by (E) identification of optogenetically tagged neurons expressing ChR2 in extracellular single-unit recordings (bottom panel). Manipulation: Stimulation (F) or inhibition (G) of neurons via light-sensitive cation channels (e.g. ChR2, top panel) or inhibitory pumps (e.g. NpHR, middle panel) provides fast, cell-type specific modulation of activity while engineered G-protein coupled receptors (Designer Receptors Activated by Designer Drugs; DREADDs, panel H) can be used to modulate activity on a longer timescale.