Fig. 2.
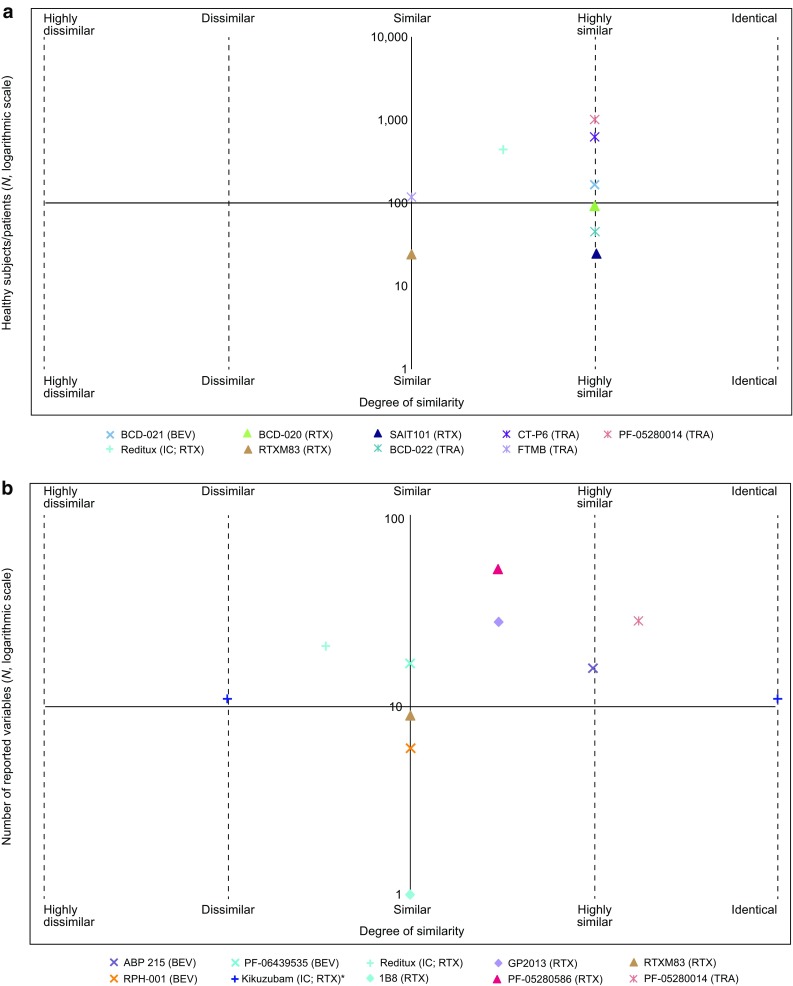
Biosimilarity and a total number of healthy subjects or patients for proposed biosimilars and ICs in clinical trials; and b breadth of data for proposed biosimilars and ICs in analytical and nonclinical studies. Totality of evidence from all available published studies (up to September 3, 2015) was used to assess “degree of similarity” for proposed biosimilars and ICs, and is based on the original conclusions made by the study authors. The scale of reference used by each author was not accounted for, as this was not uniformly reported in the literature. * based on author interpretation of study data, Kikuzubam® exhibits, in some cases, dissimilar and, in other cases, identical physicochemical characteristics compared with the originator. BEV bevacizumab, IC intended copy, RTX rituximab, TRA trastuzumab
