Figure 9.
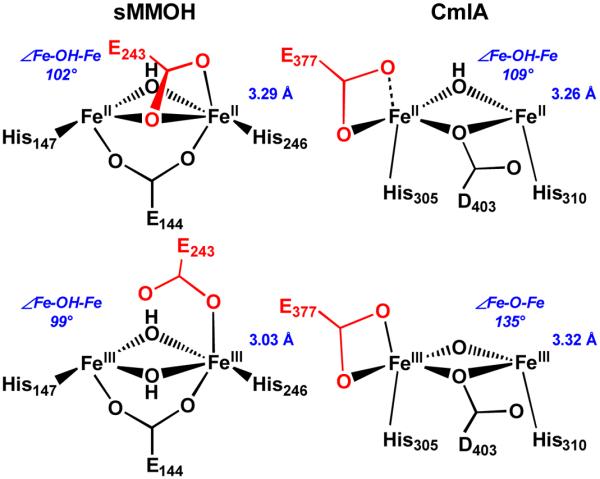
Diiron core differences between CmlA and sMMOH upon redox change. Active site models adapted from crystallographic data from refs5, 40, 65, 66. Top row: diferrous Fe centers. Bottom row: diferric Fe centers. Left: Fe•••Fe distance contracts while maintaining ∠Fe-O-Fe in sMMOH. Right: ∠Fe-O-Fe increases while maintaining Fe•••Fe distance in CmlA. Distances from EXAFS data from refs19, 58, 67. ∠Fe-O-Fe in italics; calculated by assuming a symmetric diiron core, where d(Fe1-O) = d(Fe2-O). Residues shown in red are proposed to shift during the respective catalytic cycles. Both enzymes have μ-1,1-carboxylato residues in the diferrous state (sMMOH E243 and CmlA D403), but only sMMOH E243 is proposed to shift. Some ligands are omitted for clarity.
