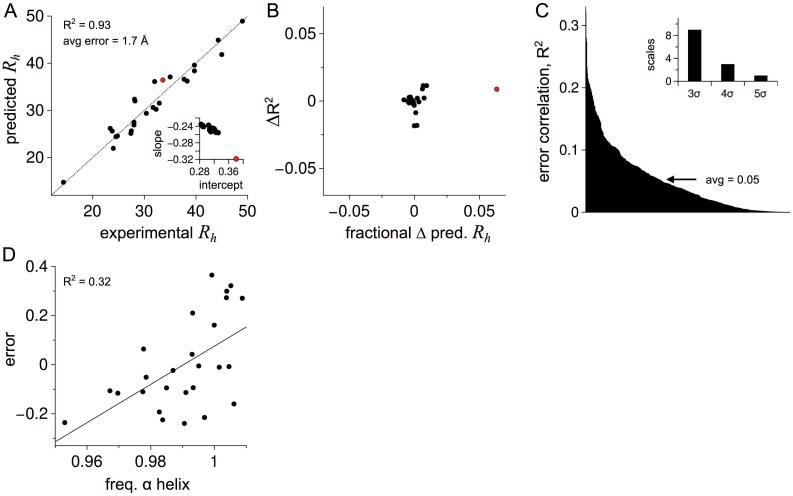
Figure 5.
Predicting Rh from intrinsic PPII propensities and net charge. A) Filled circles show Rh (in Å) predicted from sequence for each dataset IDP using equation 6. The identity line is shown by the stippled line. Inset: slope and y-intercept for the linear trend in Fig. 4C when a singular IDP is removed from the training set. B) Each filled circle represents the removal of a singular IDP from the training set. The concomitant change in correlation for predicted and experimental Rh (ΔR2) is compared to the fractional change in predicted Rh for the removed IDP. C) Prediction error, normalized for IDP size by equation 5, was compared to 567 amino acid scales (same scales as Fig. 4B). The correlation (R2) for each comparison is in rank order from left to right. Inset: Number of amino acids scales with R2 greater than the average plus 3, 4, and 5 times the standard deviation (σ). D) Comparison of prediction error to the best performing scale, normalized frequency of α helix in all α class,104 showing the trend line.