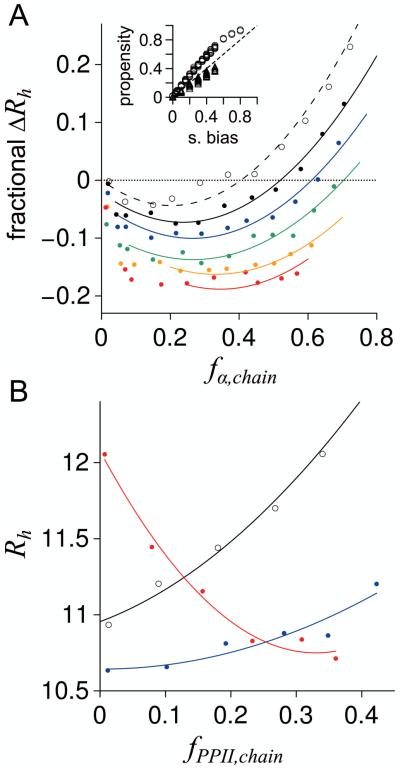
Figure 6.
Rh for poly-ALA (N = 25) simulated with intrinsic propensities for α helix and PPII. A) Open circles are ensembles with no applied sampling bias for PPII. Filled circles are ensembles simulated with applied PPII sampling biases of 0.10 (black), 0.20 (blue), 0.30 (green), 0.40 (orange), and 0.50 (red). Shown is the change in Rh relative to Rh expected from fPPII,chain using equation 4. Curves in both panels (A and B) represent data fits to second order polynomials and have no physical meaning; they were provided to highlight trends. Inset: Chain propensities calculated for each ensemble, fα,chain (circles) and fPPII,chain (triangles), and compared to the applied sampling bias. The stippled line is the identity line. B) Open circles are ensembles with no applied sampling bias for α helix. Filled circles are ensembles simulated with applied α helix bias resulting in fα,chain of ~ 0.05 – 0.07 (blue) and fα,chain of ~ 0.50 – 0.60 (red).