Abstract
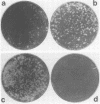
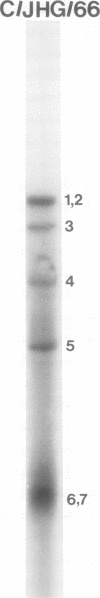
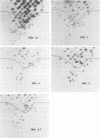
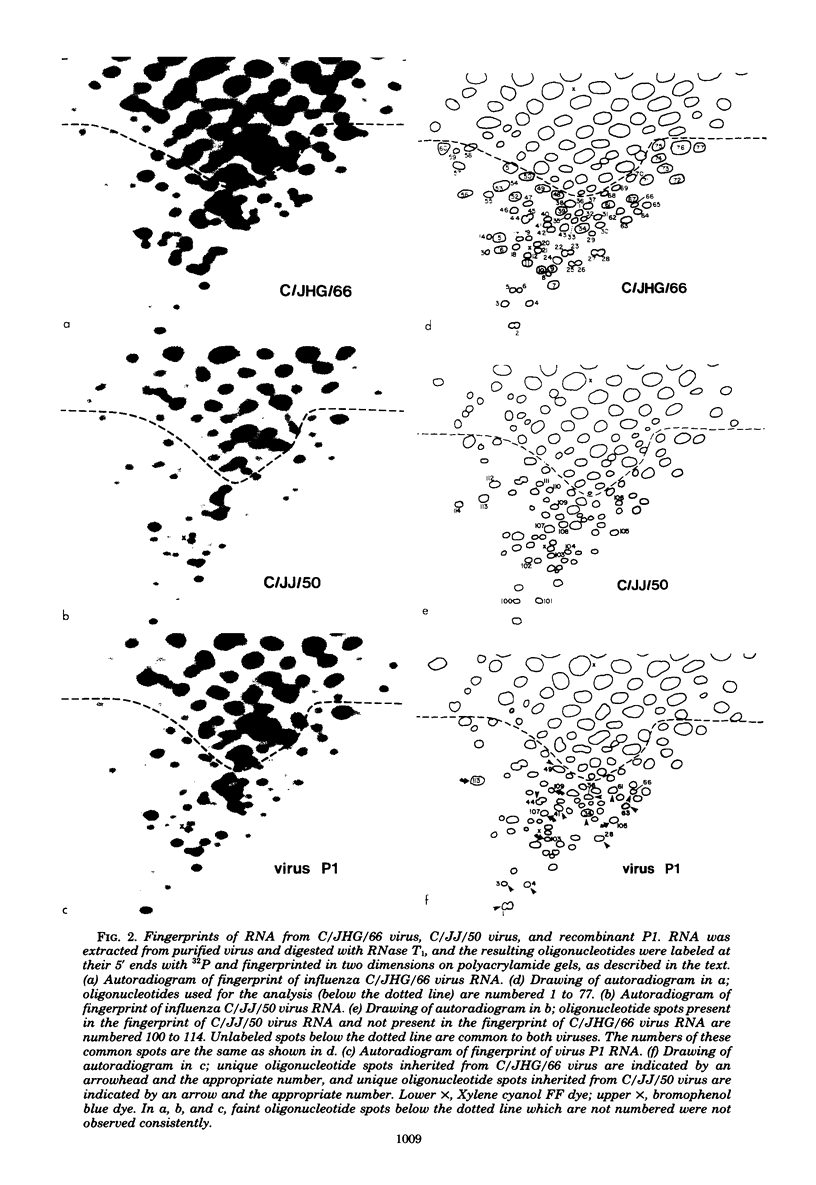
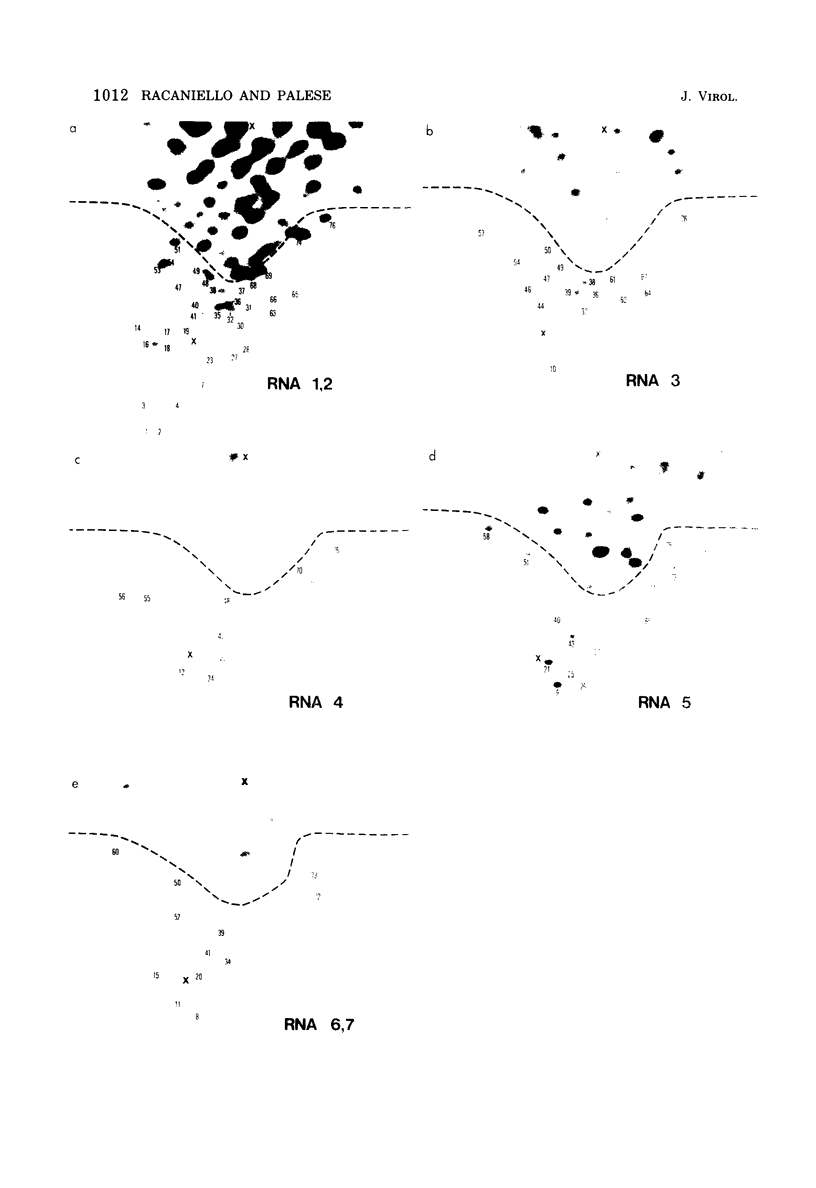
Recombinants between two different influenza C viruses were isolated. In MDCK (canine kidney) cells, one strain, C/JJ/50, caused lytic plaques, whereas C/JHG/66 virus did not produce clear plaques. From a mixed infection of MDCK cells with C/JHG/66 virus and UV-inactivated C/JJ/50 virus, clones were isolated which possessed the clear-plaque phenotype. Fingerprint analyses indicated that the RNAs of parent viruses had different oligonucleotide patterns and that one of the clones derived from the mixed infection was formed by reassortment of parental genes. This recombinant clone most likely inherited RNAs 1, 2, 3, 6, and 7 from C/JGH/66 virus and RNAs 4 and 5 from C/JJ/50 virus.
Full text
PDF

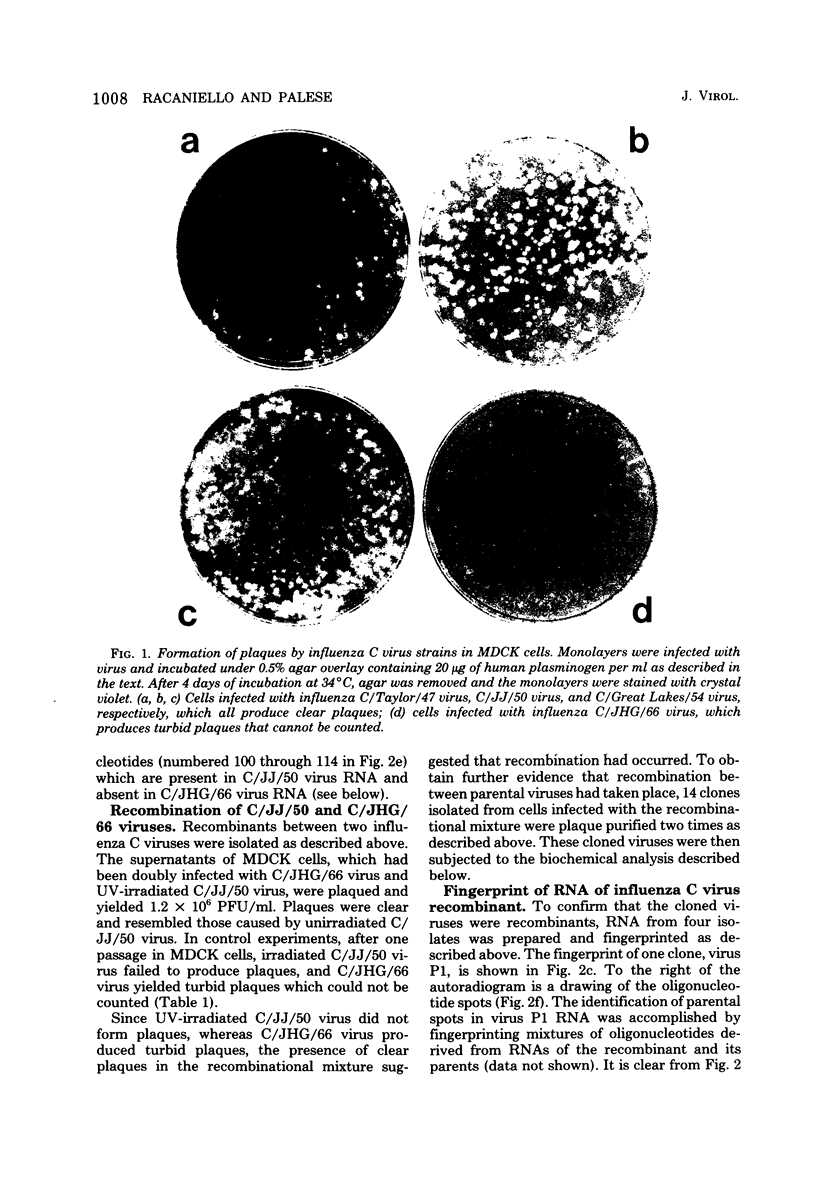




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolov K., Flewett T. H. Further observations on the structure of influenza viruses A and C. J Gen Virol. 1969 Apr;4(3):365–370. doi: 10.1099/0022-1317-4-3-365. [DOI] [PubMed] [Google Scholar]
- Archetti I., Jemolo A., Steve-Bocciarelli D., Arangio-Ruiz G., Tangucci F. On the fine structure of influenza viruses. Arch Gesamte Virusforsch. 1967;20(1):133–136. doi: 10.1007/BF01245776. [DOI] [PubMed] [Google Scholar]
- Austin M. A., Monto A. S., Maassab H. F. Growth characteristics of influenza virus type C in avian hosts. Brief report. Arch Virol. 1978;58(4):349–353. doi: 10.1007/BF01317827. [DOI] [PubMed] [Google Scholar]
- Chakraverty P. The detection and multiplication of influenza C virus in tissue culture. J Gen Virol. 1974 Dec;25(3):421–425. doi: 10.1099/0022-1317-25-3-421. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Bishop D. H., Meier-Ewert H. Structural components of influenza C virions. J Virol. 1977 Feb;21(2):658–665. doi: 10.1128/jvi.21.2.658-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N. J., Kendal A. P. Presence of a segmented single-stranded RNA genome in influenza C virus. Virology. 1976 Oct 1;74(1):239–241. doi: 10.1016/0042-6822(76)90147-1. [DOI] [PubMed] [Google Scholar]
- FRANCIS T., Jr, QUILLIGAN J. J., Jr, MINUSE E. Identification of another epidemic respiratory disease. Science. 1950 Oct 27;112(2913):495–497. doi: 10.1126/science.112.2913.495. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Apostolov K. A reticular structure in the wall of influenza C virus. J Gen Virol. 1967 Jul;1(3):297–304. [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaush C. R., Smith T. F. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol. 1968 Apr;16(4):588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G. K. The relationship of the receptors of a new strain of virus to those of the mumps-NDV-influenza group. J Exp Med. 1950 Feb;91(2):177–184. doi: 10.1084/jem.91.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G., Barrell B. G., Sanger F., Coulson A. R. Nucleotide sequences of two fragments from the coat-protein cistron of bacteriophage R17 ribonucleic acid. Biochem J. 1972 Aug;128(5):993–1006. doi: 10.1042/bj1280993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Compans R. W., Choppin W. P. An electron microscopic study of the presence or absence of neuraminic acid in enveloped viruses. Virology. 1970 Dec;42(4):1158–1162. doi: 10.1016/0042-6822(70)90368-5. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Herrler G., Petri T., Meier-Ewert H., Compans R. W. Carbohydrate components of influenza C virions. J Virol. 1979 Mar;29(3):997–1005. doi: 10.1128/jvi.29.3.997-1005.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerome K., Ishida M., Nakayama M. Absence of neuraminidase from influenza C virus. Arch Virol. 1976;50(3):241–244. doi: 10.1007/BF01320578. [DOI] [PubMed] [Google Scholar]
- Nerome K., Ishida M. The multiplication of an influenza C virus in an established line of canine kidney (MDCK) cells. J Gen Virol. 1978 Apr;39(1):179–181. doi: 10.1099/0022-1317-39-1-179. [DOI] [PubMed] [Google Scholar]
- O'Callaghan R. J., Loughlin M., Labat D. D., Howe C. Properties of influenza C virus grown in cell culture. J Virol. 1977 Dec;24(3):875–882. doi: 10.1128/jvi.24.3.875-882.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Palese P. Influenza B virus genome: assignment of viral polypeptides to RNA segments. J Virol. 1979 Jan;29(1):361–373. doi: 10.1128/jvi.29.1.361-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. M. A further note on 1233 influenza C virus. Arch Gesamte Virusforsch. 1951;4(4):485–500. doi: 10.1007/BF01241168. [DOI] [PubMed] [Google Scholar]
- Tobita K., Kilbourne E. D. Genetic recombination for antigenic markers of antigenically different strains of influenza B virus. J Virol. 1974 Feb;13(2):347–352. doi: 10.1128/jvi.13.2.347-352.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol Immunol. 1975 Dec 30;162(1):23–27. doi: 10.1007/BF02123574. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- WATERSON A. P., HURRELL J. M., JENSEN K. E. The fine structure of influenza A, B and C viruses. Arch Gesamte Virusforsch. 1963;12:487–495. doi: 10.1007/BF01242156. [DOI] [PubMed] [Google Scholar]