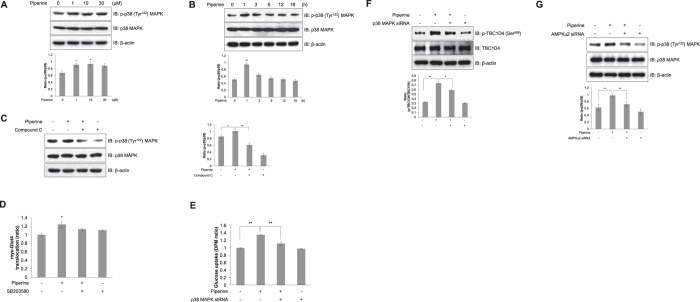
Figure 4. Piperine activates the p38 MAPK pathway in an AMPK-dependent manner.
(A) C2C12 cells were treated with different concentrations of piperine for 3 h, (B) with 30 μM piperine for the duration of time indicated, or (C) with 30 μM piperine for 3 h in the presence of 5 μM compound C. The cells were lysed, and the phosphorylation of p38 MAPK was quantified by Western blot using antibodies specific for the phosphorylated protein. The levels of total p38 MAPK and β-actin were also measured as controls for protein loading. (D) Myoblasts stably expressing L6-Glut4-myc were differentiated for 7 days, pretreated with SB203580 (5 μM) for 30 min, and then incubated with piperine for 3 h. Cell surface expression of Myc-Glut4 was quantified using an antibody-coupled colorimetric absorbance assay. (E) Differentiated L6 myotubes were transiently transfected with p38 MAPK siRNA (100 nM) for 48 hours and then treated with 30 μM piperine before 2-deoxy-d[H3]-glucose (2-DG) uptake was measured. (F) C2C12 cells were transfected with p38 MAPK siRNA (100 nM) for 2 days and then treated with piperine (30 μM) for 1 h. The cells were lysed, and the phosphorylation of TBC1D4 was quantified by Western blot analysis. The level of TBC1D4 was measured as a control for protein loading. Results from three independently replicated experiments. (G) C2C12 cells were transfected with AMPKα2 siRNA (100 nM) for 2 days and then treated with piperine (30 μM) for 1 h. The cells were lysed, and the phosphorylation of p38 MAPK was quantified by Western blot analysis. The level of p38 MAPK was measured as a control for protein loading. Results from three independently replicated experiments. *P < 0.05, **P < 0.01 compared with the untreated cells. Results from three independently replicated experiments. Blots were displayed in cropped format. Cropped images of full-length blots are shown.