Abstract
Background
The milk fat globule membrane (MFGM) is primarily composed of polar phospho- and sphingolipids, which have established biological effects on neuroplasticity. The present study aimed to investigate the effect of dietary MFGM supplementation on the neuromuscular system during post-natal development.
Methods
Growing rats received dietary supplementation with bovine-derived MFGM mixtures consisting of complex milk lipids (CML), beta serum concentrate (BSC) or a complex milk lipid concentrate (CMLc) (which lacks MFGM proteins) from post-natal day 10 to day 70.
Results
Supplementation with MFGM mixtures enriched in polar lipids (BSC and CMLc, but not CML) increased the plasma phosphatidylcholine (PC) concentration, with no effect on plasma phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidylserine (PS) or sphingomyelin (SM). In contrast, muscle PC was reduced in rats receiving supplementation with both BSC and CMLc, whereas muscle PI, PE, PS and SM remained unchanged. Rats receiving BSC and CMLc (but not CML) displayed a slow-to-fast muscle fibre type profile shift (MyHCI → MyHCIIa) that was associated with elevated expression of genes involved in myogenic differentiation (myogenic regulatory factors) and relatively fast fibre type specialisation (Myh2 and Nfatc4). Expression of neuromuscular development genes, including nerve cell markers, components of the synaptogenic agrin–LRP4 pathway and acetylcholine receptor subunits, was also increased in muscle of rats supplemented with BSC and CMLc (but not CML).
Conclusions
These findings demonstrate that dietary supplementation with bovine-derived MFGM mixtures enriched in polar lipids can promote neuromuscular development during post-natal growth in rats, leading to shifts in adult muscle phenotype.
Electronic supplementary material
The online version of this article (doi:10.1186/s12986-017-0161-y) contains supplementary material, which is available to authorized users.
Keywords: Muscle, Nerve, Myogenesis, Complex milk lipids, Agrin
Background
Skeletal muscle tissue comprises a spectrum of muscle fibre types that differ in structure, molecular composition and functional properties [1, 2]. The fibre type profile of a given muscle is initially established during embryonic development, independently of neural influences, by the differentiation of distinct myoblast lineages, which are committed to form different myofibre type populations [2, 3]. However, nerve activity subsequently plays a major role in the maintenance and modulation of the muscle fibre type profile during early post-natal development and throughout adult life [4–6]. Muscle fibre characteristics may be altered, changing size and transitioning from a fast-to-slow or a slow-to-fast fibre type profile both during normal post-natal development and in the adult organism in response to exercise, disuse and ageing [6–9]. Nutrition is an additional known, but less well understood, determinant of muscle fibre type profile [8].
Early post-natal life is a period of heightened neuromuscular plasticity, in which marked morphological changes occur in pre- and post-synaptic components of the neuromuscular junction (NMJ) [10]. These changes coincide with shifts in muscle fibre type profile as specific muscles develop their adult phenotype [2]. During embryonic development, acetylcholine (ACh) receptors (AChRs) accumulate in a broad band in the middle portion of the myofibre and extensive innervation occurs, generating polyinnervated NMJs with large motor units [11]. Subsequent maturation of the NMJ during post-natal development involves extensive reorganisation of pre- and post-synaptic morphology and a removal of surplus axonal terminals at polyinnervated NMJs. The compound agrin, released from pre-synaptic axon nerve terminals, plays a key role in NMJ maturation during post-natal development by stimulating clustering and aggregation of AChRs on the muscle fibre membrane. Multiple alternatively spliced forms of agrin that differ in their binding characteristics and bioactivity are synthesized by both nerve and muscle cells [12]. Neurons specifically express splice variants of agrin (Z+ isoforms) which include sequences encoded by the alternate exons 32 and/or 33 [12, 13]). Notably, these Z+ isoforms are 1,000-fold more active than those lacking Z exons (Z− isoforms) in inducing aggregation of AChR clusters at the NMJ [14]. Synaptic agrin is cleaved and inactivated by neurotrypsin, resulting in the release of C-terminal agrin fragments (CAFs). Plasma CAFs have been found to be elevated in a subset of human sarcopenia patients, suggesting that uncontrolled degradation of agrin at the NMJ may contribute to age-associated neuromuscular decline [15–18]. Similarly, overexpression of neurotrypsin resulted in a precocious sarcopenic phenotype that was characterised by weakness, NMJ fragmentation and pathological muscle abnormalities (termed SARCO mice) [19]. Administration of a recombinant neurotrypsin-resistant form of agrin has been shown to improve muscle pathology by minimising the disassembly of the NMJ in SARCO mice [20]. Therefore, modulation of muscle agrin signalling and/or NMJs may be a novel treatment of sarcopenia and neuromuscular disease.
Milk is the main source of nutrition in newborn mammals. It also contains a number of minor constituents that possess potential bioactivity beyond their pure nutritional significance [21, 22]. Milk fat is secreted as lipid droplets consisting of a hydrophobic triglyceride core enclosed within a thin membrane that is composed of a lipid and protein trilayer and is known as the milk fat/lipid globule membrane (MFGM) [22]. The MFGM contains polar lipids including phospholipids (PLs) [phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI)], sphingolipids [sphingomyelin (SM)] and gangliosides (GM3 and GD3) [21–23]. Although PLs and sphingolipids represent only 0.5–1% of the total lipids in milk, there is considerable interest in these compounds due to their potential bioactivity and because several health-promoting effects have been attributed to MFGM composites [21, 22, 24, 25]. In particular, MFGM composites including PLs [24], sphingolipids [26, 27] and gangliosides [28–30] have purported positive effects on neuroplasticity. For example, dietary supplementation with bovine-derived MFGM mixtures has recently been reported to promote post-natal neurodevelopment [31–35] and to limit age-associated neural decline in elderly animals [36].
Given the close relationship between nerve and muscle, MFGM supplementation may have potential to indirectly influence muscle tissue, secondary to effects on neuroplasticity. Additionally, components enriched in the MFGM, including PLs and sphingolipids, have recently been implicated as molecules that play an important direct positive role in muscle cell growth and development [37–39]. In a recent study, combined habitual exercise and dietary supplementation with a bovine-derived MFGM supplement containing both MFGM lipids and MFGM proteins was reported to minimise age-associated loss of muscle mass and force-generating capacity in senescence-accelerated mice (SAMP1 mice) [40]. Furthermore, several recent human clinical trials have reported apparent potential beneficial effects of dietary MFGM supplementation on neuromuscular function when combined with exercise training [41–43]. However, whether dietary supplementation with MFGM mixtures has direct effects on skeletal muscle plasticity, the specific MFGM components that exert bioactivity and the underlying mechanisms involved remains unclear.
In the present study, we examined the effect of dietary supplementation with bovine-derived MFGM mixtures, differing in the composition of PLs/sphingolipids and presence of MFGM proteins, during post-natal development on neuromuscular plasticity in growing rats. Specifically, we analysed the effect of feeding MFGM during development on the resulting adult muscle phenotype and the expression of the myogenic, synaptogenic and fibre type specialisation genes that are central to neuromuscular development.
Methods
Animal experiments
Experiments were approved by the Animal Ethics Committee, The University of Auckland (001260). Eight independent litters of Wistar rats were received from the animal resource unit of the University of Auckland at post-natal (PN) day 7. The litter size was normalised at birth to eight male pups per litter. At PN day 10, the pups were randomly allocated to receive dietary supplementation with blank gelatin (BG) or gelatin cubes formulated with one of three different bovine-derived MFGM mixtures (details below) (supplied by Fonterra Co-operative Group Limited). The pups were weaned at PN day 21 and experiments were terminated at PN day 70.
MFGM formulation and dietary supplementation
Gelatin cubes were used for administration of the dietary supplements, which were formulated using raspberry flavouring and gelatin (10% w/v) containing sucrose (10% w/v) as previously described [32, 33, 36, 44]. Briefly, the MFGM mixtures were dissolved in water using a food processor and were then mixed with 1 L of gelatin/sucrose mixture at 50 °C. The mixture was then transferred into ice cube trays and was firmed at 4 °C. Each cube contained 12.5 mL of gelatin/sucrose solution, with or without an added MFGM mixture. Three different MFGM mixtures were tested; they were administered at various absolute doses so as to match the administered amount of gangliosides (GD3), an established brain bioactive molecule [35]. The bovine-derived MFGM supplements tested included a complex milk lipid (CML) mixture (reported previously in [33, 35, 44]), beta serum concentrate (BSC) (reported previously in 32) and a polar-lipid-enriched complex milk lipid concentrate (CMLc, “PGC80”) that lacks an MFGM protein component (reported previously in 36). The dose of CML used and the standardised ganglioside concentration were based on our previously published study in which developing rats received dietary supplementation with CML containing 5.9% gangliosides, at a dose of 1% w/w of dietary intake [35]. The gross compositions of the three different MFGM mixtures are described in Table 1.
Table 1.
Compositions of the supplements (g/100 g)
| CML | BSC | CMLc | |
|---|---|---|---|
| Protein | 7.0 | 52.3 | 0.0 |
| MFGM protein present | N | Y | N |
| MFGM structure present | N | Y | N |
| Total fat | 80.0 | 36.2 | 88.0 |
| PLs | 45.2 | 13.7 | 71.7 |
| PC (% total PLs) | 14.5 | 27.0 | 23.1 |
| PE (% total PLs) | 27.2 | 29.2 | 34.7 |
| PI (% total PLs) | 18.7 | 8.8 | 8.8 |
| PS (% total PLs) | 26.1 | 12.1 | 12.0 |
| Ganglioside GD3 | 4.8 | 0.63 | 1.8 |
| PL:GD3 ratio | 9.4 | 21.7 | 39.8 |
| Lactose | 3.0 | 6.6 | 3.2 |
| Minerals | 8.0 | 5.2 | 9.1 |
| PLs (mg/g/day) | =0.64 × 45.2/100 (0.29) | =5.05 × 13.7/100 (0.69) | =1.78 × 71.7/100 (1.28) |
CML complex milk lipids, BSC beta serum concentrate, CMLc complex milk lipid concentrate, MFGM milk fat globule membrane, PL phospholipid, PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol, PS phosphatidylserine, SM sphingomyelin
A total of 64 animals (eight pups from eight independent litters) were studied (n = 16 per group). Treatment was performed within litter, to minimise the effect of between-litter variability. On PN day 10, two pups within each litter of eight were randomly allocated into one of the four treatment groups. The rats were hand fed from PN day 10 until PN day 21 (weaning day) and were individually cage fed thereafter until PN day 69 (week 10). The dose of MFGM supplementation was calculated daily based on animal body weight. The doses of CML, BSC and CMLc were 0.64, 5.05 and 1.78 mg/g/day respectively. The control group was fed with BG cubes, which were prepared identically except without the addition of any MFGM mixture. The rats were single caged with the allocated gel treatment for 1–2 h each day and were returned to their home cage after they had consumed the gels.
Tissue collections
On PN day 70, the rats were deeply anaesthetised with pentobarbital (125 mg/kg, i.p.) and blood samples (1–2 mL) were collected via cardiac punctures using heparin as an anticoagulant. Blood plasma was separated by centrifugation prior to storage at −80 °C. The rats were transcardially perfused with normal saline until the outflow from the heart ran clear. The posterior musculature of the lower left hind limb (triceps surae) was dissected and the soleus muscle was removed intact. The soleus muscle was weighed and then cut in half transversely at the mid belly. One portion of the soleus was snap frozen in liquid nitrogen and stored at −80 °C for molecular analysis. The remaining half was covered in Cryoglue optimum cutting temperature (OCT) compound (SLEE medical, Mainz, Germany) and rapidly frozen in dry-ice-cooled isopentane for subsequent immunohistological analysis.
Immunohistochemical analysis of muscle tissue
Cross-sections (10 μm) were cut from the mid belly of OCT-embedded soleus muscles in a cryostat at −20 °C (Leica CM3050 S, Leica Biosystems, Nussloch, Germany). Tissue sections were adhered to SuperfrostTM Plus slides (Thermo Fisher Scientific, Waltham, MA, USA) and were air-dried at room temperature. The slides were blocked in 10% goat serum (GS; Vector Laboratories, Burlingame, CA, USA) in phosphate buffered saline (PBS) at room temperature for 2 h, prior to overnight incubation with a cocktail of immunoglobulin (Ig) sub-class specific mouse monoclonal primary antibodies against MyHCI (BA-F8, mouse IgG2b, diluted 1:12.5), MyHCIIa (SC-71, mouse IgG1, 1:600) and MyHCIIb (BF-F3, mouse IgM, 1:25) as previously described [45]. An antibody against laminin (2E8, mouse IgG2a, 1:50) was used simultaneously to stain the basal lamina surrounding each myofibre. Monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA, USA. This method allows for visualisation of all four rodent MyHC isoforms simultaneously, together with the muscle fibre boundaries, on a single tissue section because MyHCIIx fibres remained unstained [45]. All primary antibodies were prepared in 10% GS solution in PBS. Following overnight primary antibody incubation, the slides were washed five times, for 5 min each time, in PBS prior to incubation with Alexa Fluor goat anti-mouse secondary antibodies (Alexa Fluor 350 IgG2b, Alexa Fluor 488 IgG1, Alexa Fluor 555 IgM and Alexa Fluor 647 IgG2a; Life Technologies, Carlsbad, CA, USA) for 2 h at room temperature. All secondary antibodies were diluted 1:500 in PBS. Following five further washes, for 5 min each time, in PBS, the slides were mounted with coverslips in Immu-Mount aqueous mounting medium (Thermo Fisher Scientific, Waltham, MA, USA). Antibody staining was visualised and images were captured at 10× magnification using an upright fluorescence microscope (Axio Imager Z2, Carl Zeiss, Oberkochen, Germany) equipped with a fully motorised automatic stage (VSlide Scanner, MetaSystems, Alltlussheim, Germany). Sequential images, spanning the entire area of the muscle cross-section, were captured and were then tiled into a single composite image using V-slide software. Global linear adjustments to the image fluorescent signal brightness and contrast were made in MetaViewer software (MetaSystems Alltlussheim, Germany). Semi-automated quantitative analysis was performed using Image J software to determine the muscle fibre type and the cross-sectional area (CSA) of individual myofibres. All fibres within the muscle section were analysed. Whilst the IHC staining method used potentially allows for the identification of hybrid myofibres the image analysis procedure used includes all fibres which express MyHCIIa myosin (including hybrid MyHCI/MyHCIIa) as Type IIa fibres. Thus, whilst the quantification procedure used does not specifically allow for analysis of the specific contribution of hybrids, any hybrid fibres present would contribute to the total MyHCIIa + fibre count.
RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from a ~ 20 mg piece of frozen soleus muscle using the RNeasy kit (Qiagen, Venlo, Netherlands) as per the manufacturer’s instructions. The RNA concentration was determined using a NanoDrop 1000 spectrophotometer. The RNA samples were diluted in nuclease-free water and first-strand cDNA synthesised from 0.5 μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA). RT-PCR was performed on a LightCycler 480 using LightCycler 480 SYBR Green I Master mix (Roche Applied Science, Indianapolis, IN, USA). The RT-PCR fluorescent emission data were analysed for the cycle threshold (Ct) value. Gapdh was selected as an appropriate housekeeping gene and was used for normalisation and quantification of the target mRNA PCR data by the 2(−∆Ct) method. A standard curve and a melting curve were performed for each primer pair to confirm efficiency and single product amplification. Primer sequences used are presented in Additional file 1: Table S1.
HPLC–MS Analysis of Plasma and Tissue PLs
Rat muscle samples (~50 mg) were homogenised in 1 mL of Milli Q water using 2.8 mm ceramic beads in a OMNI Bead Ruptor Homogeniser (Omni International, Kennesaw, GA, USA) (5.65 m/s, 2 × 1 min). Lipids were extracted from the rat muscle homogenate and plasma samples (0.25 mL) using the modified Svennerholm and Fredman [46] extraction protocol as described by Norris et al. [47]. The final non-polar chloroform fraction containing the PLs was made to 5 mL in choloroform/methanol (2:1) and used for PL analysis. The analysis of PLs was performed on an ACQUITY UPLC system (Waters, Milford, MA, USA), equipped with an APS-2 Hypersil column (150 mm × 2.1 mm, 3 μm, Thermo Electron Corporation, Waltham, MA, USA), and interfaced to a TSQ Quantum mass spectrometer (Thermo Electron Corporation, Waltham, MA, USA) using a heated electrospray ionisation source as previously described [44]. The PLs were detected using precursor ion or neutral losses that occur to the PLs during fragmentation [48].
Data analysis
The data were assessed for normality using the Shapiro–Wilk test. Variables that were found not to be normally distributed were transformed (log or square root) in order to achieve the assumption of normality. Data between the four supplementation groups were compared using a one-way analysis of variance (ANOVA). Following a significant main ANOVA effect, Holm-Šidák post-hoc tests were performed to compare each of the experimental groups with the control (BG) group. The PL compositions of plasma and muscle tissue were analysed by two-way ANOVA with supplementation group and PL species class as factors. The data are presented as means ± SEM. p < 0.05 was considered to be statistically significant.
Results
Body and muscle weights
There was no significant effect of MFGM mixture supplementation group on animal body weight, soleus muscle weight or soleus weight when normalised to body weight (mg/g body weight).
Plasma and muscle PL composition
The plasma PL concentration showed a significant interaction effect (p < 0.001) between the MFGM supplementation group and the type of PL species (Fig. 1a). PC was by far the most abundant PL species detected in plasma and was present at significantly higher concentrations compared with the other PL species (Fig. 1a). The plasma PC concentration was futher increased above that in the BG group in both the BSC group (p < 0.01) and the CMLc group (p < 0.001) but not the CML group (Fig. 1a). The plasma concentrations of other PLs, including PI, PE and SM, were not significantly influenced by the MFGM supplementation group (Fig. 1a). However, plasma lyso-phosphatidylcholine (L-PC) was found to be elevated in the CML group (p < 0.05) but not in the BSC and CMLc groups (Fig. 1a). Analysis of the PL composition of skeletal muscle tissue samples showed main effects of supplementation group (p < 0.01) and PL species (p < 0.001) (Fig. 1b). Similar to plasma, PC was the predominant PL species that was detected in muscle, although, unlike in plasma, PE was also found to be a major component within the muscle tissue homgenates (Fig. 1b). Compared with the BG group, the intramuscular concentration (μg/mg) of PC was significantly lower in the BSC group (p < 0.01) and the CMLc group (p < 0.01) (Fig. 1b). In contrast, no effect of CML supplementation on the muscle PC concentration was apparent. The muscle concentrations of PI, PE, PS and SM were not influenced by dietary supplementation with any of the three MFGM mixtures (Fig. 1b).
Fig. 1.
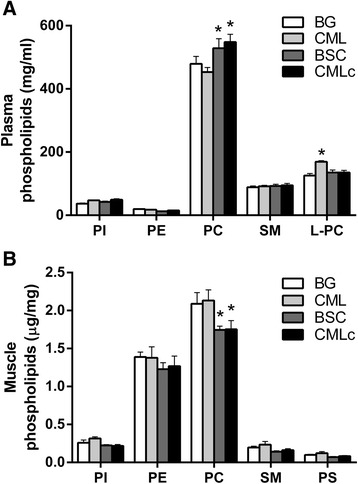
Muscle and plasma phospholipid (PL) concentrations in rats receiving dietary supplementation with various milk fat globule membrane (MFGM) mixtures. a Plasma concentration (mg/mL) of PL species. b Muscle concentration (μg/mg wet weight) of PL species
Muscle fibre type composition
There was a significant effect of supplementation group on the absolute number of MyHCIIa-positive myofibres within the soleus muscle (p < 0.05) (Fig. 2b). Similarly, there was a significant effect of supplementation group on the MyHCIIa fibre number expressed as the percentage of total fibres analysed (% composition of MyHCIIa-positive fibres) (p < 0.05) (Fig. 2c). Post-hoc analysis revealed an increase in both the absolute number (Fig. 2b) and the % composition (Fig. 2c) of MyHCIIa fibres in rats supplemented with the BSC and CMLc MFGM mixtures. In contrast, there was no significant effect of the CML MFGM mixture on the muscle fibre type composition. These data indicate a “slow-to-fast” fibre type shift from Type I (MyHCI-positive) towards Type IIa (MyHCIIa-positive) muscle fibres in the soleus muscle of rats receiving supplementation with the BSC and CMLc MFGM mixtures but not the CML MFGM mixture.
Fig. 2.
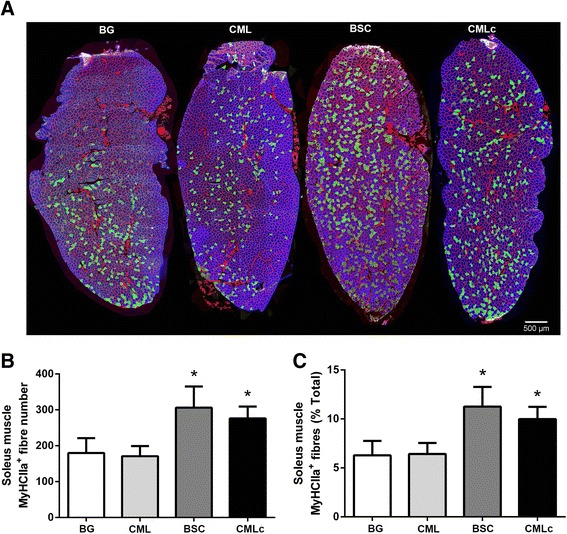
Muscle fibre type composition of the soleus muscle of rats supplemented with various milk fat globule membrane (MFGM) mixtures during post-natal development. a Representative images of soleus muscle cross-sections probed with mouse monoclonal antibodies against MyHCI (IgG2b, Alexa Fluor 350, blue), MyHCIIa (IgG1, Alexa Fluor 488, green), MyHCIIb (IgM, Alexa Fluor 555, red) and laminin (IgG2a, Alexa Fluor 647, far-red, pseudo coloured red). b Total number of soleus muscle fibres staining positive for MyHCIIa. c Percentage composition of total myofibres staining positive for MyHCIIa. * p < 0.05 vs BG group
Muscle fibre size
There was no significant effect of supplementation with any of the three MFGM mixtures on the CSA of Type I (MyHCI-positive) (Fig. 3b) or Type IIA (MyHCIIa-positive) myofibres (Fig. 3c). These data suggest that supplementation with MFGM components did not alter the soleus muscle fibre size.
Fig. 3.
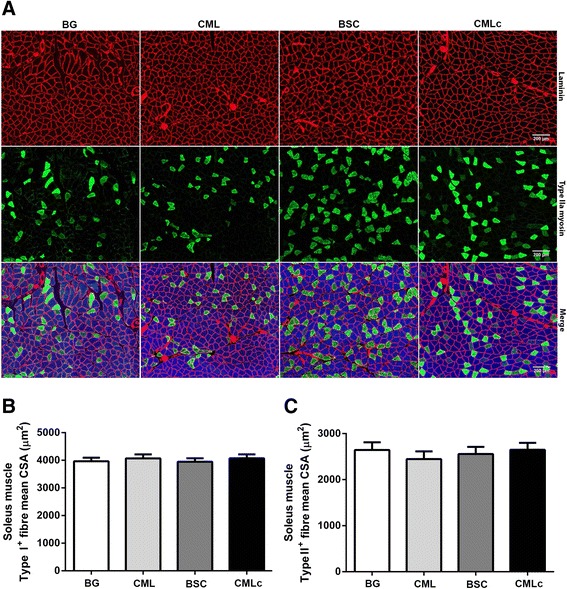
Soleus muscle myofibre cross-sectional area (CSA) (μm2) in rats supplemented with various milk fat globule membrane (MFGM) mixtures. a Representative images of soleus muscle cross-sections probed with mouse monoclonal antibodies against MyHCI (IgG2b, Alexa Fluor 350, blue), MyHCIIa (IgG1, Alexa Fluor 488, green), MyHCIIb (IgM, Alexa Fluor 555, red) and laminin (IgG2a, Alexa Fluor 647, far-red, pseudo coloured red). b Mean CSA (μm2) of soleus muscle Type I (MyHCI-positive) myofibres. c Mean CSA (μm2) of soleus muscle Type IIa (MyHCIIa-positive) myofibres
Skeletal muscle mrna expression
Contractile protein genes
As expected, mRNA expression of genes encoding the slow-twitch MyHCI (Myh7) and intermediate-fast MyHCIIa (Myh2) isoforms was abundant in the predominantly tetanic slow-twitch soleus muscle. Expression of mRNA encoding the fast-twitch rodent myosin isoforms MyHCIIx (Myh1) and MyHCIIb (Myh4) was low and highly variable in this muscle (Fig. 4a). There were significant between-group differences in mRNA expression of Myh2 in the soleus by one-way ANOVA (p < 0.05). Post-hoc analysis showed that Myh2 expression was increased in the CMLc group compared with the BG group (p < 0.05). A similar trend towards elevated expression of Myh2 was observed for the BSC group, but this failed to achieve statistical significance (p = 0.08 vs BG). Supplementation with the CML MFGM mixture had no effect on Myh2 mRNA expression. There was no effect of any of the three MFGM mixtures on mRNA expression of the slow-twitch MyHCI isoform (Myh7), nor on the expression of troponin I type 1 (skeletal, slow) (Tnni) or troponin I type 2 (skeletal, fast) (Tnni2) (Fig. 4a).
Fig. 4.
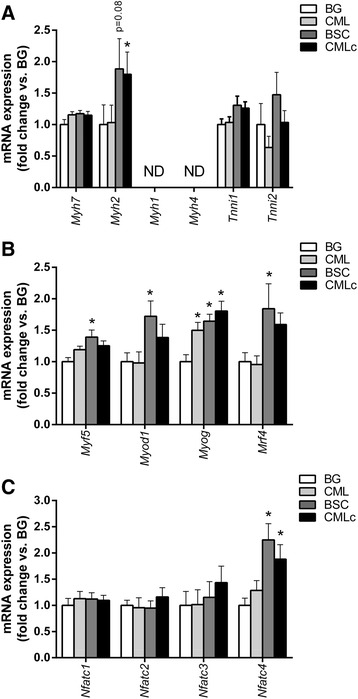
Dietary supplementation with lipid-enriched milk fat globule membrane (MFGM) mixtures influences muscle gene expression. a mRNA expression of transcripts encoding muscle contractile proteins in the soleus of rats receiving dietary supplementation with blank gel (BG), complex milk lipids (CML), beta serum concentrate (BSC) or complex milk lipd concentrate (CMLc). b mRNA expression of myogenic regulatory factor (MRF) genes in the soleus muscle of rats receiving dietary supplementation with BG, CML, BSC or CMLc. c mRNA expression of NFAT isoforms in the soleus muscle of rats receiving dietary supplementation with BG, CML, BSC or CMLc. Values are mean ± SEM. * p < 0.05 vs BG group
Myogenic regulatory factors
To further explore the mechanisms by which MFGM supplementation may influence the skeletal muscle phenotype, we measured the expression of the myogenic regulatory factor (MRF) genes that are involved in skeletal muscle development and satellite cell myogenesis in mature muscle tissue (Fig. 4b). There were significant between-group differences by one-way ANOVA for muscle mRNA expression of all four MRF family members including Myf5 (p < 0.05), Myod1 (p < 0.05), Myog (p < 0.001) and Myf6 (p < 0.05) (Fig. 4b). Post-hoc tests revealed that Myog mRNA levels were higher in the CML (p < 0.001), BSC (p < 0.001) and CMLc (p < 0.001) groups compared with the BG group. However, expression of Myf5 (p < 0.01), Myod1 (p < 0.05) and Myf6 (p = 0.05) was significantly increased only in the BSC group. Similar trends towards elevated expression of Myf5 (p = 0.06 vs BG) and Myf6 (p = 0.16 vs BG) were observed in the CMLc group, but these failed to reach statistical significance.
Fibre type specialisation genes
Compared with the BG group, mRNA expression of Nfatc4 was elevated in both the BSC group (p < 0.001) and the CMLc group (p < 0.01) (but not the CML group) (Fig. 4c). No difference between groups was observed for mRNA expression of the other NFAT isoforms, including Nfatc1, Nfatc2 and Nfatc3.
Neuromuscular gene expression
Synaptogenic genes
mRNA expression of components of the agrin–LRP4–MuSK signalling pathway, including Agrn (p < 0.001), Lrp4 (p < 0.05), Musk (p < 0.001) and Rapsn (p < 0.05), were found to be significantly different between the supplementation groups by one-way ANOVA (Fig. 5A). Post-hoc analysis showed that dietary supplementation with both BSC and CMLc increased the expression of Agrn (both p < 0.01), Lrp4 (the Agrn receptor) (both p < 0.05) and Musk (CMLc p < 0.001, BSC p < 0.05). However, only the CMLc group displayed elevated muscle expression of Rapsn. Unlike supplementation with BSC and CMLc, supplementation with the CML mixture had no significant effect on the expression of LRP4–MuSK signalling pathway components.
Fig. 5.
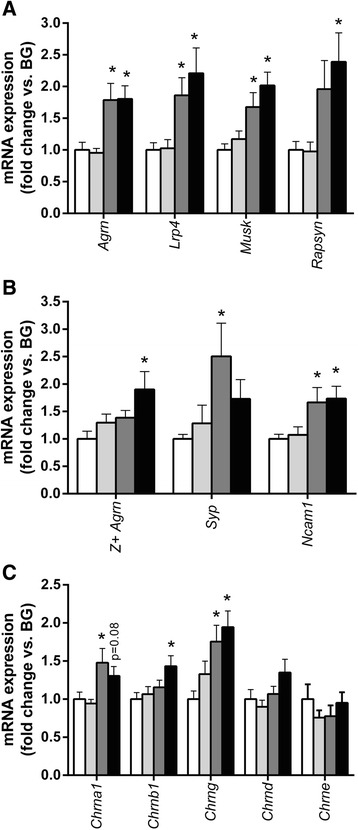
Milk fat globule membrane (MFGM) supplementation influences neuromuscular gene expression. a mRNA expression of components of the agrin–LRP4 pathway in the soleus muscle of rats receiving dietary supplementation with BG, CML, BSC or CMLc. b mRNA expression of nerve cell markers in the soleus muscle of rats receiving dietary supplementation with blank gel (BG), complex milk lipids (CML), beta serum concentrate (BSC) or complex milk lipid concentrate (CMLc). c mRNA expression of acetylcholine receptor (AChR) subunits in the soleus muscle of rats receiving dietary supplementation with BG, CML, BSC or CMLc. Values are mean ± SEM. * p < 0.05 vs BG group
Pre-synaptic components
The Agrn primer pair used above (Agrn 1 F: TTCCTCAGCAACTACAAACCTG, Agrn 3R: TTCACACACAGCACCAAAGC) amplify a region of rat Agrn mRNA spanning exons 1 to 3 ubiquitous to all Agrn splice variants (both muscle Z- and nerve Z+ isoforms). In order to determine whether mRNA of the nerve derived Z+ Agrn isoforms mRNA (which possess substantially greater AChR cluster aggregation bioactivity) could be detected within whole skeletal muscle samples, and whether apparent differences in Agrn expression were exclusively attributable to modulation of Z− Agrn isoforms known to be expressed by myofibres themselves, we designed primers to specifically amplify agrin mRNA splice variants that include exon 32 (corresponding to protein isoforms Z8 and Z19) [49]. RT-PCR reactions with a forward primer specific for exon 30 (Agrn 30 F: TGTCCTGGGGGCTTCTCTGG) in combination with a reverse located within exon 32 (Agrn 32R: CTGGGATCTCATTGGTCAGCTC) present only in the nerve derived Z + 8 (containing exon 32 but lacking exon 33) and Z + 19 (containing both exons 32 and 33) splice variants demonstrated amplification of rat muscle tissue cDNA samples not observed in RT− or non-template control samples (Fig. 5b, Additional file 2: Figure S1). Agarose gel (4%) electrophoresis of the amplified RT-PCR products revealed bands corresponding to the expected sizes of 102 bp with the Agrn 1 F/Agrn 3R (pan Agrn) primer pair and 149 bp with Agrn 30 F/Agrn 32R (Z+ Agrn) primer pair. Sequencing of these bands following gel extraction/purification confirmed that the 102 bp product generated with Agrn 1 F/Agrn 3R primers aligned with a region of rat Agrn between exons 1–3 and the 149 bp product with Agrn 30 F/Agrn 32R aligned with region located within alternatively spliced exon 32 (CGAGCTGACCAATGAGATCCCAG) present only in the nerve derived Z+ isoforms (Additional file 2: Figure S1). Real time RT-PCR reactions with the Agrn 30 F/Agrn 32R primer pair showed that the expression of Z+ Agrn mRNA within muscle tissue significantly differed between supplementation groups by one-way ANOVA (p < 0.05) (Fig. 5b). Post-hoc analysis demonstrated significantly elevated muscle Z+ Agrn mRNA expression in the CMLc group when compared to the BG group. Neither CML nor BSC supplementation had any significant effect on the expression of Z+ Agrn (Fig. 5b).
Consistent with our observation of detectable expression of nerve derived Z+ Agrn mRNA in whole muscle tissue; mRNA of the synaptic vesicle protein synaptophysin (Syp) was also apparently detected. Agarose gel (4%) electrophoresis of the amplified product from the real time PCR reactions (Syp F: TTTGCTACGTGTGGCAGCTA, Syp R: ACACTTGGTGCAGCCTGAAG) showed a band corresponding to the expected size of 121 bp. Sequencing of the 121 bp band following gel extraction/purification confirmed that the amplified product aligned with rat Syp mRNA (Additional file 2: Figure S2). Real time RT-PCR reactions with the Syp F/Syp R primer pair revealed that muscle Syp expression was significantly different between groups by one-way ANOVA (p < 0.05) with post-hoc analysis showing significantly elevated Syp in BSC supplemented animals. Finally, soleus mRNA expression of neural cell adhesion molecule (Ncam1) was also significantly different between groups (p < 0.01). Ncam1 was elevated in the BSC (p < 0.05) and CMLc (p < 0.05) groups compared with the BG group (Fig. 5a). In contrast, Ncam1 expression did not differ between the CML and BG groups.
Post-synaptic neuromuscular components
mRNA expression of AChR subunits alpha 1 (Chrna1) (p < 0.01), beta (Chrnb1) (p < 0.05) and gamma (Chrng) (p < 0.01) was significantly different between the supplementation groups (Fig. 5c). AChR delta (Chrnd) also showed a similar trend towards between-group differences, but this failed to reach statistical significance (p = 0.09). Post-hoc analysis revealed that Chrng mRNA was elevated in both the BSC and CMLc groups when compared with the BG group. Expression of Chrna1 was also elevated only in the BSC group, although the CMLc group exhibited a similar trend (p = 0.08 vs BG). Only the CMLc group exhibited significantly increased muscle mRNA expression of Chrnb1. Expression of the AChR epsilon subunit (Chrne) did not differ between the MFGM supplementation groups.
Discussion
The current study assessed the effect of dietary supplementation with different bovine-derived MFGM mixtures on post-natal neuromuscular development in growing rats. None of the MFGM supplements tested here had any effect on soleus muscle mass or myofibre size. However, a slow-to-fast shift, i.e. Type I towards Type IIa muscle fibres was observed in rats supplemented with both BSC and CMLc, which occurred as a result of an increase in both the absolute number and the relative concentration of Type IIa fibres. The effect of BSC and CMLc supplementation on muscle phenotype was associated with elevated expression of genes involved in myogenesis (MRFs), fibre type specialisation (Myh2 and NFATc4) and neuromuscular plasticity/synaptogenesis (agrin–LRP4 pathway). These findings show that dietary supplementation with bovine-derived MFGM mixtures that are enriched in polar lipids promotes neuromuscular development in rats.
Skeletal muscles are still immature at birth and important changes in the muscle fibre type profile, which coincide with maturation of the neuromuscular system, take place during early post-natal development, including the disappearance of polyneuronal innervation and the acquisition of specific motor neuron firing patterns [2]. The development and the maintenance of the fibre type profile of tetanic slow-twitch muscles (e.g. the soleus) are particularly dependent on nerve activity. In the current study, rats that received dietary supplementation with BSC and CMLc (but not CML) displayed a greater proportion of muscle fibres staining positive for the MyHCIIa isoform within the soleus muscle at PN day 70. RT-PCR analysis of muscle gene expression suggested that this effect appeared to be primarily attributable to elevated expression of the intermediate-fast MyHCIIa isoform (Myh2 gene), with no indication of suppressed expression of the slow-twitch MyHCI isoform (Myh7 gene). We also measured expression of the skeletal muscle contractile protein isoforms slow troponin (Tnni1) and fast troponin (Tnni2), but found that they were not influenced by MFGM supplementation. These data suggest that dietary supplementation with specific MFGM mixtures during post-natal development leads to shifts in the adult muscle fibre type profile that are primarily attributable to upregulation of the intermediate-fast Type IIa myosin isoform. An increase in the percentage of Type IIa myofibres within the soleus muscle would potentially be expected to lead to changes in muscle function including an increased force generating potential. Although no measures of muscle function were undertaken in the current study, our soleus muscle fibre type composition results may explain the previously reported positive effect of MFGM on tetanic contractile force of the soleus muscle in mice [40]. The soleus muscle is a prototypical slow-twitch muscle consisting primarily of Type I myofibres and thus a slow-to-fast fibre type shift could be seen to disrupt the normal profile of the soleus with the potential for possible negative effects on oxidative capacity. Nevertheless, Satoshi et al. 2014 reported that mice receiving MFGM supplementation displayed increased muscular endurance capacity in association with increased soleus muscle mRNA expression of oxidative genes including peroxisome proliferator-activated receptor-γ coactivator 1α (Pgc1α) and Cpt-1b [50].
Previous studies that have investigated the effect of nutrition on skeletal muscle fibre type have utilised models of energy surplus or deficit including high-fat-diet-induced obesity and caloric restriction [8]. In response to short term feeding of rodents with a high fat diet, a number of studies have reported a fast-to-slow fibre type profile shift in skeletal muscle [51–55], associated with a concurrent increase in muscle oxidative capacity [56–58]. In contrast, a single recent study reported slow-to-fast muscle fibre type transformation in response to a more prolonged (1 year) high fat diet in mice [59]. In the present study, we observed an increase in the absolute number and the relative proportion of Type IIa myofibres in the soleus muscle of rats fed BSC and CMLc, despite very little difference in overall caloric intake and macronutrient composition of the four diets. This suggests that supplementation with specific MFGM mixtures enriched in polar lipids exerted bioactive effects on the muscle fibre type profile independent of caloric/nutritional value. MFGM contains numerous potentially bioactive molecules including polar lipids as well as specific membrane proteins [21–23]. As all three tested MFGM supplements were matched for ganglioside content in the present study (and yet CML was without effect), it appears to be unlikely that the effects observed are related to the dietary intake of gangliosides. Furthermore, BSC is a complex mixture of ingredients, including a substantial MFGM protein component, which is lacking in the more purified CMLc mixture. Despite this, overall, the effects of BSC and CMLc were very similar. Thus, overall, the effects observed here also do not appear to be primarily attributable to a bioactive MFGM protein component. Although further investigation into the bioactive composites is required, collectively, these lines of evidence suggest that the polar lipid composition of bovine MFGM is a likely soure of bioactivity.
Phospholipids and sphingolipids have established bioactive properties related to neuroplasticity as well as muscle cell growth and development [37–39]. For example, cell surface exposure of PS on myoblasts is an indispensable event for myoblast fusion [60, 61] and treatment with PS liposomes can enhance muscle fibre formation in vitro [62]. Conversely, ablation of the PS receptor has been found to impair muscle regeneration following injury in mice [63, 64]. SM levels also relate to the state of activation of muscle myogenic precursor cells (satellite cells) [65], with SM metabolism to the metabolite sphingosine-1-phosphate (S1P) being a crucial pathway in satellite cell activation/proliferation [66–70], myoblast migration [71] and myogenic differentiation [72–77]. Furthermore, in vivo treatment with S1P has been shown to protect against contraction-induced muscle fatigue [78], minimise denervation-induced muscle atrophy [79] and enhance skeletal muscle regenerative capacity following traumatic injury [80]/damaging muscle contractions (exercise) [81]. Consistent with a positive role of polar lipids in the regulation of muscle cell growth and development, in the current study, we found that dietary supplementation with PL-enriched MFGM mixtures increased muscle expression of the MRFs including Myf5, Myod1, Myog and Mrf4. The MRFs play a crucial role in embryonic myogenesis, but also play multiple distinct roles in post-natal muscle, including developmental growth, fibre type control and growth/regeneration of adult myofibres in response to injury caused by mechanical loading. For example, Myod1 is expressed more highly in fast-twitch fibres than in slow-twitch fibres and Myod1-null mice show shifts in fibre type of fast muscle towards a slower phenotype [82, 83]. Conversely, overexpression of an active form of Myod1 in muscle results in a slow-to-fast fibre type conversion [84].
Nuclear factor of activated T cell (NFAT) family members are known to play a major role in converting fast and slow motor neuron stimulation patterns into specific transcriptional programs that drive muscle fibre type specialisation [85, 86].We detected expression Nfatc1, Nfatc2, Nfatc3 and Nfatc4 mRNA in adult soleus muscle, but found that only Nfatc4 was induced in response to MFGM (BSC and CMLc) supplementation. An indispensable role of Nfatc1 in the maintenance of a slow muscle phenotype is well established [86, 87]. However, more recent work has shown that, depending on the applied nerve activity pattern, different combinations of NFAT isoform expression contributes to the transcription of muscle fibre type specific genes [85]. For example, transcription of MyHC genes in the predominantly fast-twitch rat extensor digitorum longus (EDL) muscle uses different combinations of NFAT isoforms, ranging from MyHC-slow, which uses all four NFAT isoforms, to MyHCIIb, which requires only Nfatc4 [85]. The precise role of Nfatc4 in fibre type specialisation of the predominantly slow-twitch soleus muscle, which generally lacks expression of the MyHCIIb isoform, is unclear. Nevertheless, the notion that Nfatc4 is specifically involved in driving the expression MyHC isoforms associated with a relatively faster muscle phenotype [85] is consistent with the Type 1 to Type IIa soleus muscle fibre type shift, concurrent to elevated Nfatc4 expression, that was observed in response to MFGM supplementation in the current study.
Multiple splice variants of agrin exist which vary via the inclusion or exclusion of exons 32 and/or 33 (nomenclature of ref 14). Whereas most cell types synthesize agrin, only neurons produce the alternatively spliced Z+ agrin isoforms that include the sequence encoded by exons 32 and/or 33 [12]. These Z+ exons encode a domain of 8–19 amino acids that confers up to a 1,000-fold increase in AChR clustering activity relative to Z- agrin [14]. Additionally, the Z8 (with 8 additional amino acids encoded by exon 32) and Z19 (with 19 amino acids encoded by exons 32 and 33) isoforms are 150-fold more potent in promoting AChR clustering than isoforms lacking exon 32 and 45-fold more potent than the Z11 isoform (including exon 33 alone) [88–90]. Using a primer pair spanning exons 1–3 of rat Agrn mRNA (which is present in all splice variants) we found that Agrn mRNA expression was increased in the muscle of rats receiving dietary supplementation with BSC or CMLc, but not CML. Since Z- agrin variants have previously been found to be the only isoforms expressed within skeletal muscle tissue [12], this finding likely reflects an effect of MFGM supplementation on muscle derived agrin. However, using primers designed to specifically amplify Agrn mRNA splice variants that include exon 32 (corresponding to protein isoforms Z8 and Z19) we were apparently also able to detect expression of the Z+ Agrn mRNA within the rat soleus muscle. Gel electrophoresis confirmed a band of the expected size which aligned with the region within spliced exon 32 following band extraction and sequencing. On the basis of previous reports [12], we can only presume that Z+ Agrn mRNA expression detected was derived from motor neuron axons and/or presynaptic motor nerve terminals located within the muscle tissue. Rats receiving CMLc supplementation appeared to display significantly increased expression of Z+ Agrn when compared to BG, suggesting increased Agrn expression in CMLc supplemented animals may be at least partially due to induction nerve Agrn. Consistent with this hypothesis was detection and modulation of mRNA expression of the pre-synaptic nerve terminal marker synaptophysin within rat muscle. Increased muscle Agrn and neural Z+ Agrn mRNA expression in the soleus muscle tissue of MFGM supplemented rats was also accompanied by elevated muscle expression of downstream components of the post-synaptic agrin–LRP4 signalling pathway, including the agrin receptor Lrp4, the associated receptor tyrosine kinase Musk and the intracellular AChR-clustering protein Rapsn. The role of agrin in the determination of muscle phenotype has been investigated recently in mice in which the levels of agrin at the NMJ were reduced by overexpression of neurotrypsin enzyme, which cleaves active agrin to form inactive CAFs (SARCO mice) [19]. Young adult SARCO mice exhibit a precocious sarcopenic phenotype that is characterised by loss of muscle force, destabilisation of NMJs and a preferential loss of fast-twitch type II myofibres [19]. Consistently, loss of agrin function as the result of a point mutation results in deterioration of the NMJ, leads to muscle fibre atrophy and leads to a fast-to-slow muscle fibre type shift [91]. Plasma CAFs have been found to be elevated in elderly humans, suggesting that they may be a useful biomarker of sarcopenia arising from age-associated neuromuscular decline [15–18]. Interestingly, injection of an agrin biologic (NT-1654) was able to reverse the sarcopenia-like phenotype in SARCO mice, suggesting that modulating the agrin–LRP4–MuSK pathway may be a novel therapeutic strategy in the treatment of age-related muscle wasting, neuromuscular disease and associated muscle dysfunction [20]. To our knowledge, the present study is the first report of the modulation of muscle agrin levels as a result of nutritional supplementation. These findings suggest that dietary ingestion of, or dietary supplementation with, bovine MFGM or composite material could be a potential therapeutic means of manipulating the agrin–LRP4 pathway in muscle tissue.
Previously, Haramizu et al. reported the effect of dietary supplementation with an MFGM mixture in combination with habitual exercise in age related muscle dysfunction using a model of senescence-accelerated mice (SAMP1 mice) [40]. SAMP1 mice that received combined MFGM and exercise exhibited increased muscle mass (quadriceps) and contractile force (soleus and EDL) [40]. Interestingly, microarray analysis of the quadriceps muscle revealed that transcripts related to the biological process of “nervous system development and function” were most differentially expressed between the MFGMEx group and the control SAMP1 group [40]. Subsequent verification by PCR analysis showed significant induction of the neuromuscular/synaptogenic genes Musk and Dok7, as well as the myogenic genes Myod1 and Myog, in response to combined MFGM supplementation and habitual exercise [40]. However, no significant differences were found in response to MFGM supplementation when administered in the absence of habitual exercise [40]. In support of the findings of Haramizu et al. [40], we report induction of several of the same genes (Myod1, Myog, Musk) or closely related genes (e.g. other MRFs and agrin–LRP4 pathway components) in muscle tissue of rats supplemented with BSC and CMLc, even in the absence of an exercise stimulus. To our knowledge, the current study is the first report of a direct effect of MFGM supplementation on the expression of genes related to nervous system development independent of an exercise stimulus. As early post-natal life is a stage of neuromuscular plasticity, direct effects of dietary MFGM supplementation on skeletal muscle tissue may be more readily observed in this model. However, in adult skeletal muscle, it is possible that a remodelling stimulus (e.g. exercise) may be necessary for MFGM supplementation to exert a modulatory bioactive effect.
Conclusion
In conclusion, we found that dietary supplementation with specific bovine-derived MFGM mixtures, including BSC and CMLc, which are enriched in polar lipid components, promotes neuromuscular development in growing rats, leading to shifts in the adult’s muscle phenotype. These findings suggest that previously reported effects of MFGM or composite molecules on neuroplasticity may extend beyond the central nervous system and may impact peripheral tissues including skeletal muscle. On the basis of these findings, ingestion of dietary sources of MFGM or supplementation with bovine-derived MFGM mixtures enriched in bioactive lipids, or isolated specific bioactive MFGM components, may exert potential positive effects on skeletal muscle structure and function in physiological settings of development, growth and muscle regeneration/remodelling (e.g. tissue injury/healing, exercise recovery, reloading following disuse). Furthermore, the apparent positive effect of MFGM supplementation on modulation of neuromuscular/synaptogenic development pathways may be indicative of a potential therapeutic benefit of bovine MFGM or composite molecules in settings of nerve-related muscle dysfunction such as ageing and neuromuscular disease.
Acknowledgements
We would like thank Jo Perry (Liggins Institute, University of Auckland) and Chantal Pileggi (Liggins Institute, University of Auckland) for their assistance with agarose gel electrophoresis, extraction/purification and sequencing of real time RT-PCR products.
Funding
This research was supported by the New Zealand Primary Growth Partnership (PGP) program, funded by Fonterra Co-operative Group Ltd. and the New Zealand Ministry for Primary Industries (MPI).
Availability of data and materials
Please contact author for data requests.
Authors’ contributions
JG, DCS, AKHM, PM, ACF, and AR, designed the experiment. JFM, BD, VCF, KL, and BYF collected the data. JFM, BD, and BYF analysed the data. JFM, VCF, JG, AF, and DCS drafted the manuscript. AKHM, ACF, AR, PM, DCS revised that manuscript. All authors approved the final version of the manuscript.
Competing interests
J.F.M., B.D., D.C.S., K.L., and J.G. received financial support from the New Zealand Primary Growth Partnership (PGP), funded by Fonterra Co-operative Group Ltd and the NZ Ministry for Primary Industries (MPI) to conduct this study. A.K.H.M., A.C.F., B.Y.F., P.M., and A.R., are current employees of Fonterra Co-operative Group Limited and were involved in design of the research and editing of the manuscript. The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
Experiments were approved by the Animal Ethics Committee, The University of Auckland (001260).
Abbreviations
- ACh
Acetylcholine
- AChR
Acetylcholine receptor
- BG
Blank gel
- BSC
Beta serum concentrate
- CAFs
C-terminal agrin fragment
- CML
Complex milk lipids
- CMLc
Complex milk lipid concentrate
- HPLC
High performance liquid chromatography
- Ig
Immunoglobulin
- IHC
Immunohistological
- MFGM
Milk fat globule membrane
- MRF
Myogenic regulatory factor
- MS
Mass spectrometry
- MyHC
Myosin heavy chain
- NMJ
Neuromuscular junction
- OCT
Optimal cutting temperature
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PI
Phosphatidylinositol
- PL
Phospholipid
- PS
Phosphatidylserine
- SAMP1 mice
Senescence –accelerated mice
- SM
Sphingomyelin
Additional files
Primer pairs used for real time RT-PCR analysis of rat soleus muscle mRNA expression. (PDF 109 kb)
Real time RT‐PCR analysis of Agrn and Z+ Agrn expression in rat soleus muscle. Figure S2. Real time RT‐PCR analysis of synaptophysin expression in rat soleus muscle. A (PDF 369 kb)
Contributor Information
James F. Markworth, Email: j.markworth@auckland.ac.nz
Brenan Durainayagam, Email: b.durainayagam@auckland.ac.nz.
Vandre C. Figueiredo, Email: v.casagrandefigueiredo@auckland.ac.nz
Karen Liu, Email: karen.liu@auckland.ac.nz.
Jian Guan, Email: j.guan@auckland.ac.nz.
Alastair K. H. MacGibbon, Email: alastair.macgibbon@fonterra.com
Bertram Y. Fong, Email: bertram.fong@fonterra.com
Aaron C. Fanning, Email: aaron.fanning@fonterra.com
Angela Rowan, Email: angela.rowan@fonterra.com.
Paul McJarrow, Email: paul.mcjarrow@fonterra.com.
David Cameron-Smith, Phone: +64-9-923-1336, Email: d.cameron-smith@auckland.ac.nz.
References
- 1.Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiologica. 2010;199:451–63. doi: 10.1111/j.1748-1716.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 2.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 3.Condon K, Silberstein L, Blau HM, Thompson WJ. Differentiation of fiber types in aneural musculature of the prenatal rat hindlimb. Dev Biol. 1990;138:275–95. doi: 10.1016/0012-1606(90)90197-Q. [DOI] [PubMed] [Google Scholar]
- 4.Gambke B, Lyons GE, Haselgrove J, Kelly AM, Rubinstein NA. Thyroidal and neural control of myosin transitions during development of rat fast and slow muscles. FEBS Lett. 1983;156:335–9. doi: 10.1016/0014-5793(83)80524-9. [DOI] [PubMed] [Google Scholar]
- 5.Gundersen K. Determination of muscle contractile properties: the importance of the nerve. Acta Physiol Scand. 1998;162:333–41. doi: 10.1046/j.1365-201X.1998.0336e.x. [DOI] [PubMed] [Google Scholar]
- 6.Schiaffino S. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology. 2007;22:269–78. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 7.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–9. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Matsakas A, Patel K. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol. 2009;24:611–29. doi: 10.14670/HH-24.611. [DOI] [PubMed] [Google Scholar]
- 9.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–72. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 10.Shi L, Fu AKY, Ip NY. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012;35:441–53. doi: 10.1016/j.tins.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–33. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoch W, Ferns M, Campanelli JT, Hall ZW, Scheller RH. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron. 1993;11:479–90. doi: 10.1016/0896-6273(93)90152-H. [DOI] [PubMed] [Google Scholar]
- 13.Rupp F, Ozcelik T, Linial M, Peterson K, Francke U, Scheller R. Structure and chromosomal localization of the mammalian agrin gene. J Neurosci. 1992;12:3535–44. doi: 10.1523/JNEUROSCI.12-09-03535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess RW, Nguyen QT, Son YJ, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron. 1999;23:33–44. doi: 10.1016/S0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 15.Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, Vrijbloed JW. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, Drey M, Sieber CC, Vrijbloed JW. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol. 2013;48:69–75. doi: 10.1016/j.exger.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Stout JR, Fragala MS, Hoffman JR, Robinson EH, 4th, McCormack WP, Townsend JR, Jatjner AR, Emerson NS, Oliveira LP, Fukuda DH. C-terminal agrin fragment is inversely related to neuromuscular fatigue in older men. Muscle Nerve. 2015;51:132–3. doi: 10.1002/mus.24443. [DOI] [PubMed] [Google Scholar]
- 18.Marzetti E, Calvani R, Lorenzi M, Marini F, D’Angelo E, Martone AM, Celi M, Tosato M, Bernabei R, Landi F. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol. 2014;60:79–82. doi: 10.1016/j.exger.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–93. doi: 10.1096/fj.11-191262. [DOI] [PubMed] [Google Scholar]
- 20.Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, Ruegg MA, Vrijbloed JW. Injection of a Soluble Fragment of Neural Agrin (NT-1654) Considerably Improves the Muscle Pathology Caused by the Disassembly of the Neuromuscular Junction. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contarini G, Povolo M. Phospholipids in milk Fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 2013;14:2808–31. doi: 10.3390/ijms14022808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontecha J, Rodriguez-Alcal LM, Calvo MV, Jurez M. Bioactive milk lipids. Curr Nutr Food Sci. 2011;7:155–9. doi: 10.2174/157340111797264804. [DOI] [Google Scholar]
- 23.Fong BY, Norris CS, MacGibbon AKH. Protein and lipid composition of bovine milk-fat-globule membrane. Int Dairy J. 2007;17:275–88. doi: 10.1016/j.idairyj.2006.05.004. [DOI] [Google Scholar]
- 24.Küllenberg D, Taylor LA, Schneider M, Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11:3. doi: 10.1186/1476-511X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitsberg VL. Invited review: bovine milk Fat globule membrane as a potential nutraceutical. J Dairy Sci. 2005;88:2289–94. doi: 10.3168/jds.S0022-0302(05)72906-4. [DOI] [PubMed] [Google Scholar]
- 26.Oshida K, Shimizu T, Takase M, Tamura Y, Shimizu T, Yamashiro Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr Res. 2003;53:589–93. doi: 10.1203/01.PDR.0000054654.73826.AC. [DOI] [PubMed] [Google Scholar]
- 27.Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–59. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 28.McJarrow P, Schnell N, Jumpsen J, Clandinin T. Influence of dietary gangliosides on neonatal brain development. Nutr Rev. 2009;67:451–63. doi: 10.1111/j.1753-4887.2009.00211.x. [DOI] [PubMed] [Google Scholar]
- 29.Ryan JM, Rice GE, Mitchell MD. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr Res. 2013;33:877–87. doi: 10.1016/j.nutres.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res. 2012;37:1230–44. doi: 10.1007/s11064-012-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer FR, Kleinman RE. An infant formula with decreased weight gain and higher IQ: are we there yet? Am J Clin Nutr. 2014;99:757–8. doi: 10.3945/ajcn.114.084798. [DOI] [PubMed] [Google Scholar]
- 32.Guan, J., A. MacGibbon, B. Fong, Z. Rong, K. Liu, A. Rowen, and P. McJarrow. 2015. Long-Term Supplementation with Beta Serum Concentrate (BSC), a Complex of Milk Lipids, during Post-Natal Brain Development Improves Memory in Rats. Nutrients In press. [DOI] [PMC free article] [PubMed]
- 33.Guillermo RB, Yang P, Vickers MH, McJarrow P, Guan J. Supplementation with complex milk lipids during brain development promotes neuroplasticity without altering myelination or vascular density. Food Nutr Res. 2015;59:25765. doi: 10.3402/fnr.v59.25765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99:860–8. doi: 10.3945/ajcn.113.064295. [DOI] [PubMed] [Google Scholar]
- 35.Vickers MH, Guan J, Gustavsson M, Krägeloh CU, Breier BH, Davison M, Fong B, Norris C, McJarrow P, Hodgkinson SC. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr Res. 2009;29:426–35. doi: 10.1016/j.nutres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Guan J, MacGibbon A, Zhang R, Elliffe DM, Moon S, Liu D-X. Supplementation of complex milk lipid concentrate (CMLc) improved the memory of aged rats. Nutr Neurosci. 2015;18:22–9. doi: 10.1179/1476830513Y.0000000096. [DOI] [PubMed] [Google Scholar]
- 37.Bruni P, Donati C. Pleiotropic effects of sphingolipids in skeletal muscle. Cell Mol Life Sci. 2008;65:3725–36. doi: 10.1007/s00018-008-8236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal. 2011;15:2501–17. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jager R, Purpura M, Kingsley M. Phospholipids and sports performance. J Int Soc Sports Nutr. 2007;4:5. doi: 10.1186/1550-2783-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haramizu S, Mori T, Yano M, Ota N, Hashizume K, Otsuka A, Hase T, Shimotoyodome A. Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice. Springerplus. 2014;3:339. doi: 10.1186/2193-1801-3-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, Hase T, Hosoi E, Yoshida H. Effects of exercise and milk Fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ota N, Soga S, Hase T, Shimotoyodome A. Daily consumption of milk fat globule membrane plus habitual exercise improves physical performance in healthy middle-aged adults. Springerplus. 2015;4:120. doi: 10.1186/s40064-015-0896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soga S, Ota N, Shimotoyodome A. Dietary milk fat globule membrane supplementation combined with regular exercise improves skeletal muscle strength in healthy adults: a randomized double-blind, placebo-controlled, crossover trial. Nutr J. 2015;14:85. doi: 10.1186/s12937-015-0073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gustavsson M, Hodgkinson SC, Fong B, Norris C, Guan J, Krageloh CU, Breier BH, Davison M, McJarrow P, Vickers MH. Maternal supplementation with a complex milk lipid mixture during pregnancy and lactation alters neonatal brain lipid composition but lacks effect on cognitive function in rats. Nutr Res. 2010;30:279–89. doi: 10.1016/j.nutres.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in Rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svennerholm L, Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980;617:97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- 47.Norris C, Fong B, MacGibbon A, McJarrow P. Analysis of phospholipids in rat brain using liquid chromatography-mass spectrometry. Lipids. 2009;44:1047–54. doi: 10.1007/s11745-009-3357-8. [DOI] [PubMed] [Google Scholar]
- 48.Fong B, Ma L, Norris C. Analysis of phospholipids in infant formulas using high performance liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2013;61:858–65. doi: 10.1021/jf304877k. [DOI] [PubMed] [Google Scholar]
- 49.Ruggiu M, Herbst R, Kim N, Jevsek M, Fak JJ, Mann MA, Fischbach G, Burden SJ, Darnell RB. Rescuing Z+ agrin splicing in Nova null mice restores synapse formation and unmasks a physiologic defect in motor neuron firing. Proc Natl Acad Sci U S A. 2009;106:3513–8. doi: 10.1073/pnas.0813112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satoshi H, Noriyasu O, Atsuko O, Kohjiro H, Satoshi S, Tadashi H, Takatoshi M, Akira S. Dietary milk fat globule membrane improves endurance capacity in mice. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1009–17. doi: 10.1152/ajpregu.00004.2014. [DOI] [PubMed] [Google Scholar]
- 51.Ciapaite J, van den Berg SA, Houten SM, Nicolay K, Willems van Dijk K, Jeneson JA. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6 J mice. J Nutr Biochem. 2015;26:155–64. doi: 10.1016/j.jnutbio.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 52.de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW-V, de Groot P, Müller M, Mariman E, Smit E. Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6 J mice. Physiol Genomics. 2008;32:360–9. doi: 10.1152/physiolgenomics.00219.2007. [DOI] [PubMed] [Google Scholar]
- 53.Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One. 2009;4:1–9. doi: 10.1371/journal.pone.0007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas MM, Trajcevski KE, Coleman SK, Jiang M, Di Michele J, O’Neill HM, Lally JS, Steinberg GR, Hawke TJ. Early oxidative shifts in mouse skeletal muscle morphology with high-fat diet consumption do not lead to functional improvements. Physiol Rep. 2014;2. [DOI] [PMC free article] [PubMed]
- 55.Trajcevski KE, O’Neill HM, Wang DC, Thomas MM, Al-Sajee D, Steinberg GR, Ceddia RB, Hawke TJ. Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoeks J, de Wilde J, Hulshof MFM, van den Berg SAA, Schaart G, van Dijk KW, Smit E, Mariman ECM. High Fat diet-induced changes in mouse muscle mitochondrial phospholipids Do Not impair mitochondrial respiration despite insulin resistance. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0027274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephenson EJ, Camera DM, Jenkins TA, Kosari S, Lee JS, Hawley JA, Stepto NK. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. Am J Physiol Endocrinol Metab. 2012;302:E1541–9. doi: 10.1152/ajpendo.00590.2011. [DOI] [PubMed] [Google Scholar]
- 58.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–92. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 59.Denies MS, Johnson J, Maliphol AB, Bruno M, Kim A, Rizvi A, Rustici K, Medler S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol Rep. 2014;2:e00204. doi: 10.1002/phy2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaspar P, Dvorak M. Involvement of phosphatidylserine externalization in the down-regulation of c-myb expression in differentiating C2C12 cells. Differentiation. 2008;76:245–52. doi: 10.1111/j.1432-0436.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 61.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, Schutte B, Borgers M, Ramaekers FC. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–42. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 62.Jeong J, Conboy IM. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem Biophys Res Commun. 2011;414:9–13. doi: 10.1016/j.bbrc.2011.08.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–7. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu SF, Baylies MK. Cell biology: death brings new life to muscle. Nature. 2013;497:196–7. doi: 10.1038/nature12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagata Y, Kobayashi H, Umeda M, Ohta N, Kawashima S, Zammit PS, Matsuda R. Sphingomyelin levels in the plasma membrane correlate with the activation state of muscle satellite cells. J Histochem Cytochem. 2006;54:375–84. doi: 10.1369/jhc.5A6675.2006. [DOI] [PubMed] [Google Scholar]
- 66.Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439–50. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Fortier M, Figeac N, White RB, Knopp P, Zammit PS. Sphingosine-1-phosphate receptor 3 influences cell cycle progression in muscle satellite cells. Dev Biol. 2013;382:504–16. doi: 10.1016/j.ydbio.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, Zhang M, Proia RL, Hoffman EP, Saba JD. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS One. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagata Y, Partridge TA, Matsuda R, Zammit PS. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol. 2006;174:245–53. doi: 10.1083/jcb.200605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapizzi E, Donati C, Cencetti F, Nincheri P, Bruni P. Sphingosine 1-phosphate differentially regulates proliferation of C2C12 reserve cells and myoblasts. Mol Cell Biochem. 2008;314:193–9. doi: 10.1007/s11010-008-9780-y. [DOI] [PubMed] [Google Scholar]
- 71.Becciolini L, Meacci E, Donati C, Cencetti F, Rapizzi E, Bruni P. Sphingosine 1-phosphate inhibits cell migration in C2C12 myoblasts. Biochim Biophys Acta. 2006;1761:43–51. doi: 10.1016/j.bbalip.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Bernacchioni C, Cencetti F, Blescia S, Donati C, Bruni P. Sphingosine kinase/sphingosine 1-phosphate axis: a new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet Muscle. 2012;2:15. doi: 10.1186/2044-5040-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de la Garza-Rodea AS, Baldwin DM, Oskouian B, Place RF, Bandhuvula P, Kumar A, Saba JD. Sphingosine phosphate lyase regulates myogenic differentiation via S1P receptor-mediated effects on myogenic microRNA expression. FASEB J. 2014;28:506–19. doi: 10.1096/fj.13-233155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2005;19:449–51. doi: 10.1096/fj.04-1780fje. [DOI] [PubMed] [Google Scholar]
- 75.Donati C, Nincheri P, Cencetti F, Rapizzi E, Farnararo M, Bruni P. Tumor necrosis factor-alpha exerts pro-myogenic action in C2C12 myoblasts via sphingosine kinase/S1P2 signaling. FEBS Lett. 2007;581:4384–8. doi: 10.1016/j.febslet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Meacci E, Nuti F, Donati C, Cencetti F, Farnararo M, Bruni P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J Cell Physiol. 2008;214:210–20. doi: 10.1002/jcp.21187. [DOI] [PubMed] [Google Scholar]
- 77.Squecco R, Sassoli C, Nuti F, Martinesi M, Chellini F, Nosi D, Zecchi-Orlandini S, Francini F, Formigli L, Meacci E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol Biol Cell. 2006;17:4896–910. doi: 10.1091/mbc.E06-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danieli-Betto D, Germinario E, Esposito A, Megighian A, Midrio M, Ravara B, Damiani E, Libera LD, Sabbadini RA, Betto R. Sphingosine 1-phosphate protects mouse extensor digitorum longus skeletal muscle during fatigue. Am J Physiol Cell Physiol. 2005;288:C1367–73. doi: 10.1152/ajpcell.00246.2004. [DOI] [PubMed] [Google Scholar]
- 79.Zanin M, Germinario E, Dalla Libera L, Sandona D, Sabbadini RA, Betto R, Danieli-Betto D. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am J Physiol Cell Physiol. 2008;294:C36–46. doi: 10.1152/ajpcell.00164.2007. [DOI] [PubMed] [Google Scholar]
- 80.Danieli-Betto D, Peron S, Germinario E, Zanin M, Sorci G, Franzoso S, Sandona D, Betto R. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am J Physiol Cell Physiol. 2010;298:C550–8. doi: 10.1152/ajpcell.00072.2009. [DOI] [PubMed] [Google Scholar]
- 81.Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J Cell Mol Med. 2011;15:2498–511. doi: 10.1111/j.1582-4934.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hughes SM, Koishi K, Rudnicki M, Maggs AM. MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mech Dev. 1997;61:151–63. doi: 10.1016/S0925-4773(96)00631-4. [DOI] [PubMed] [Google Scholar]
- 83.Macharia R, Otto A, Valasek P, Patel K. Neuromuscular junction morphology, fiber-type proportions, and satellite-cell proliferation rates are altered in MyoD(−/−) mice. Muscle Nerve. 2010;42:38–52. doi: 10.1002/mus.21637. [DOI] [PubMed] [Google Scholar]
- 84.Ekmark M, Rana ZA, Stewart G, Hardie DG, Gundersen K. De-phosphorylation of MyoD is linking nerve-evoked activity to fast myosin heavy chain expression in rodent adult skeletal muscle. J Physiol. 2007;584:637–50. doi: 10.1113/jphysiol.2007.141457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA. 2009;106:13335–40. doi: 10.1073/pnas.0812911106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCullagh KJA, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, Lømo T, Schiaffino S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA. 2004;101:10590–5. doi: 10.1073/pnas.0308035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but. Proc Natl Acad Sci USA. 2001;98:13108. doi: 10.1073/pnas.231148598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-I. [DOI] [PubMed] [Google Scholar]
- 89.Ferns M, Hoch W, Campanelli JT, Rupp F, Hall ZW, Scheller RH. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 1992;8:1079–86. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- 90.Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–36. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bogdanik LP, Burgess RW. A valid mouse model of AGRIN-associated congenital myasthenic syndrome. Hum Mol Genet. 2011;20:4617–33. doi: 10.1093/hmg/ddr396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.


