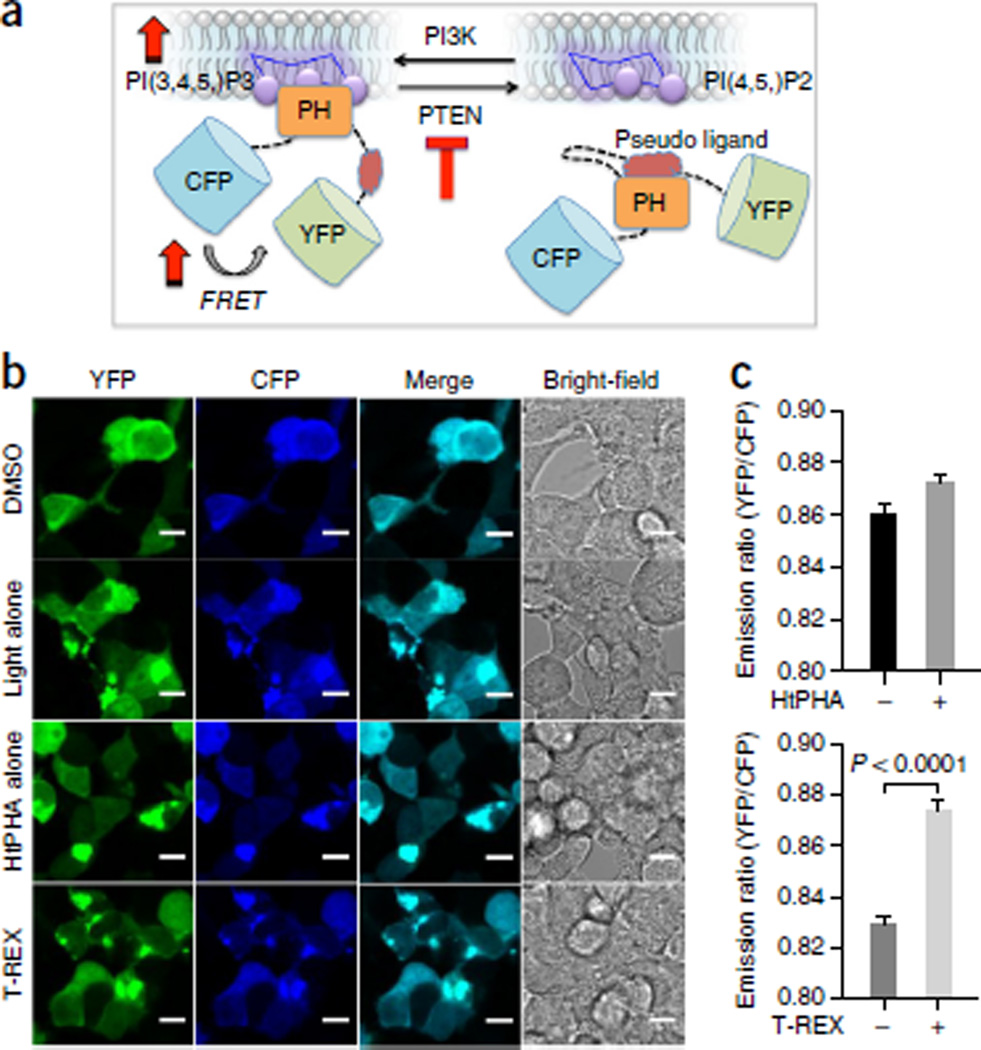
Figure 9.
FRET-based biosensor assay in live cells, reporting the levels of endogenous PIP3 subsequent to PTEN-targeted redox modification enabled by T-REX. (a) Live HEK-293 cells expressing ‘lnPAkt’ FRET biosensor88 and HaloPTEN were subjected to T-REX conditions that enabled substoichiometric HNEylation of PTEN55. Dominant loss-of-function inactivation of PTEN upregulates the membrane-bound PIP3 phosphoinositide. Increase in cellular PIP3 competitively binds the pleckstrin homology (PH) domain of Akt, displacing the ‘pseudo ligand’88. Conformational change associated with the membrane recruitment results in an increase in FRET signal88. (b) Representative live-cell images and (c) quantification of the YFP:CFP emission ratio. Scale bars, 20 µm. Top: Control: HtPHA treatment alone did not perturb the emission ratio appreciably. Bottom: T-REX redox targeting of PTEN selectively enhances FRET signal (right bar) as compared with that of samples exposed to light alone (left bar). Error bars designate the standard error of the mean (N = 170 cells). a adapted with permission from Ananthanarayanan B., Ni, Q. & Zhang, J. Signal propagation from membrane messengers to nuclear effectors revealed by reporters of phosphoinositide dynamics and Akt activity. Proc. Natl. Acad. Sci. USA 102, 15081–15086 (2005). Copyright 2005 National Academy of Sciences, USA.