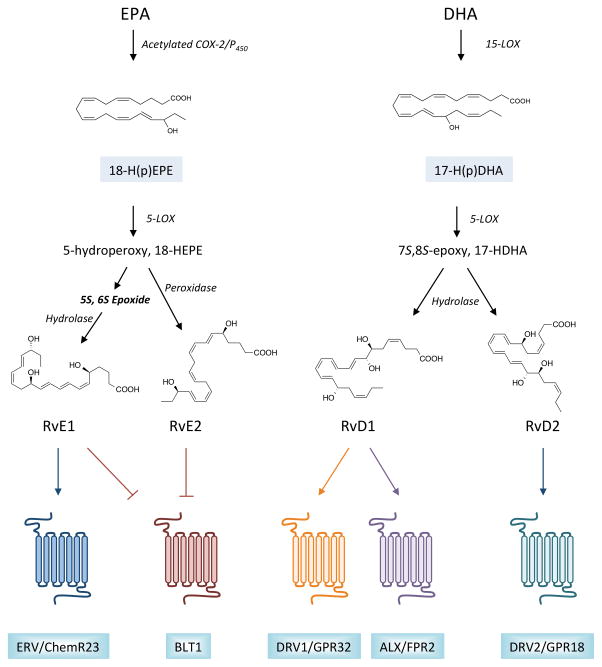
Figure 2. Biosynthesis and receptors for resolvins.
The family of resolvins is divided primarily into two groups based on the omega-3 polyunsaturated fatty acid (PUFA) from which they are formed. For E-series resolvin biosynthesis, eicosapentaenoic acid (EPA) is utilized as a substrate for acetylated COX-2 or cytochrome P450 enzymes, giving rise to a 18-hydroperoxide intermediate that can be converted to either RvE1 or RvE2 by 5-LOX and additional enzymatic transformations, as indicated. Similarly, DHA serves as a substrate for 15-LOX, giving rise to a 17-hydroperoxide intermediate. This intermediate is subsequently converted to RvD1 or RvD2 via 5-LOX, the formation of an epoxide intermediate, and subsequent enzymatic hydrolysis. Monohydroxy fatty acids, 18-HEPE and 17-HDHA, serve as pathway markers for E-series resolvins and D-series resolvins, respectively. Once formed, resolvins elicit their potent biological actions by activating specific G-protein coupled receptors (GPCRs) in a stereoselective manner. RvE1 is an agonist for ERV/ChemR23, while both RvE1 and RvE2 block the actions of pro-inflammatory leukotriene B4 by binding to its receptor, BLT1. D-series resolvins are agonists for distinct GPCRs, with RvD1 binding to DRV1/GPR32 and ALX/FPR2, and RvD2 binding to GPR18.