Abstract
Aim:
Accumulation of α-synuclein (α-syn) in the brain is a characteristic of Parkinson's disease (PD). In this study, we investigated whether treatment with tunicamycin, an endoplasmic reticulum (ER) stress inducer, led to the accumulation of α-syn in PC12 cells, and where α-syn protein was accumulated, and finally, whether bibenzyl compound 20c, a novel compound isolated from Gastrodia elata (Tian ma), could alleviate the accumulation of α-syn and ER stress activation in tunicamycin-treated PC12 cells.
Methods:
PC12 cells were treated with tunicamycin for different time (6 h, 12 h, 24 h, 48 h). Cell viability was determined by a MTT assay. Subcellular fractions of ER and mitochondria were extracted with the Tissue Endoplasmic reticulum Isolation Kit. The levels of α-syn protein and ER-stress-associated downstream chaperones were detected using Western blots and immunofluorescence.
Results:
Treatment of PC12 cells with tunicamycin (0.5–10 μg/mL) dose-dependently increased the accumulation of α-syn monomer (19 kDa) and oligomer (55 kDa), and decreased the cell viability. Accumulation of the two forms of α-syn was observed in both the ER and mitochondria with increasing treatment time. Co-treatment with 20c (10−5 mol/L) significantly increased the viability of tunicamycin-treated cells, reduced the level of α-syn protein and suppressed ER stress activation in the cells, evidenced by the reductions in phosphorylation of eIF2α and expression of spliced ATF6 and XBP1.
Conclusion:
Tunicamycin treatment caused accumulation of α-syn monomer and oligomer in PC12 cells. Bibenzyl compound 20c reduces the accumulation of α-syn and inhibits the activation of ER stress, which protected PC12 cells against the toxicity induced by tunicamycin.
Keywords: Gastrodia elata, bibenzyl compound 20c, PC12 cells, tunicamycin, α-synuclein, ER stress, Parkinson's disease, neuroprotection
Introduction
Parkinson's disease (PD) is an irreversible neurodegenerative disease. Lewy bodies (LBs) and Lewy neuritis (LNs) are the hallmarks of PD pathogenesis1,2. Fibrillar aggregates of ubiquitinated, phosphorylated, and/or S-nitrosylated forms of α-syn are major components of LBs and LNs3. Numerous studies have suggested that the accumulation of pathological proteins in the brain is a common characteristic of most neurodegenerative diseases, including PD, Alzheimer's disease (AD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS)4. These neurodegenerative diseases are classified as protein-misfolding disorders (PMDs). Endoplasmic reticulum (ER) dysfunction is suggested to be one of the primary shared characteristics of these disorders3. The accumulation of misfolded proteins is sensed by ER resident molecules, which initiate the expression of target genes, impact ER capacity5 and activate ER stress. Subsequently, a complex signaling pathway to regulate cell responses to ER stress called the unfolded protein response (UPR) is initiated. The initiation of the UPR directly activates three central transmembrane ER signaling chaperones, including PRKP-like ER kinase (PERK), inositol-requiring kinase 1 (IRE1) and activating transcription factor 6 (ATF6). These signaling pathways increase the expression of ER chaperones, inhibit protein entry into ER and accelerate the degradation of retrograde misfolded proteins, which can ameliorate the accumulation of misfolded proteins in the ER6,7. The activation of the PERK pathway promotes the phosphorylation of eIF2α and the expression of transcription factor ATF4, which increases the expression of ER chaperones to reduce protein entry into the ER. During ER stress, ATF6 translocates to the Golgi and releases transcription factors to migrate into the nucleus and regulate gene expression. When IRE1 is activated, it catalyzes the splicing of the mRNA encoding the X-box-binding protein 1 (XBP1) to produce XBP1 and regulate target genes8.
Although the mechanisms of PD pathogenesis remain unclear, many studies have indicated that the accumulation of α-syn may be a neurotoxic factor9,10. However, the mechanisms underlying α-syn accumulation and how α-syn accumulation contributes to neurodegeneration remain poorly understood. Studies have indicated that sodium butyrate, which is an ER stress inducer, can induce an increase in the oligomeric forms of α-syn in 3D5 cells, and this effect was blocked by co-treatment with the ER stress inhibitor salubrinal, which suggested that ER stress could promote the aggregation of α-syn in 3D5 cells1. However, whether ER stress is neurotoxic to PC12 cells is still unknown. Furthermore, the impact of ER stress on the accumulation of the monomeric and oligomeric forms of α-syn and the location of α-syn during ER stress remain unclear.
Gastrodia elata (Tian ma) is a traditional herb that is used to treat headaches, hypertension and neurodegenerative diseases. Recent studies have found that treatment with this herb can enhance cognitive function and help prevent oxidation11,12,13. The compound 20c (2-[4-hydroxy-3-(4-hydroxybenzyl)-4-(4-hydroxybenzyl) phenol) (Figure 3C) was isolated from Gastrodia elata and is a novel bibenzyl compound. Based on previous data from our laboratory, 20c can protect PC12 cells against damage induced by rotenone, which suggests that 20c is a compound with potential neuroprotective effects against PD (data not shown). However, the impact of 20c on the accumulation of α-syn has yet to be determined, and no evidence has been reported that reveals the impact of 20c on the activation of ER stress.
Figure 3.
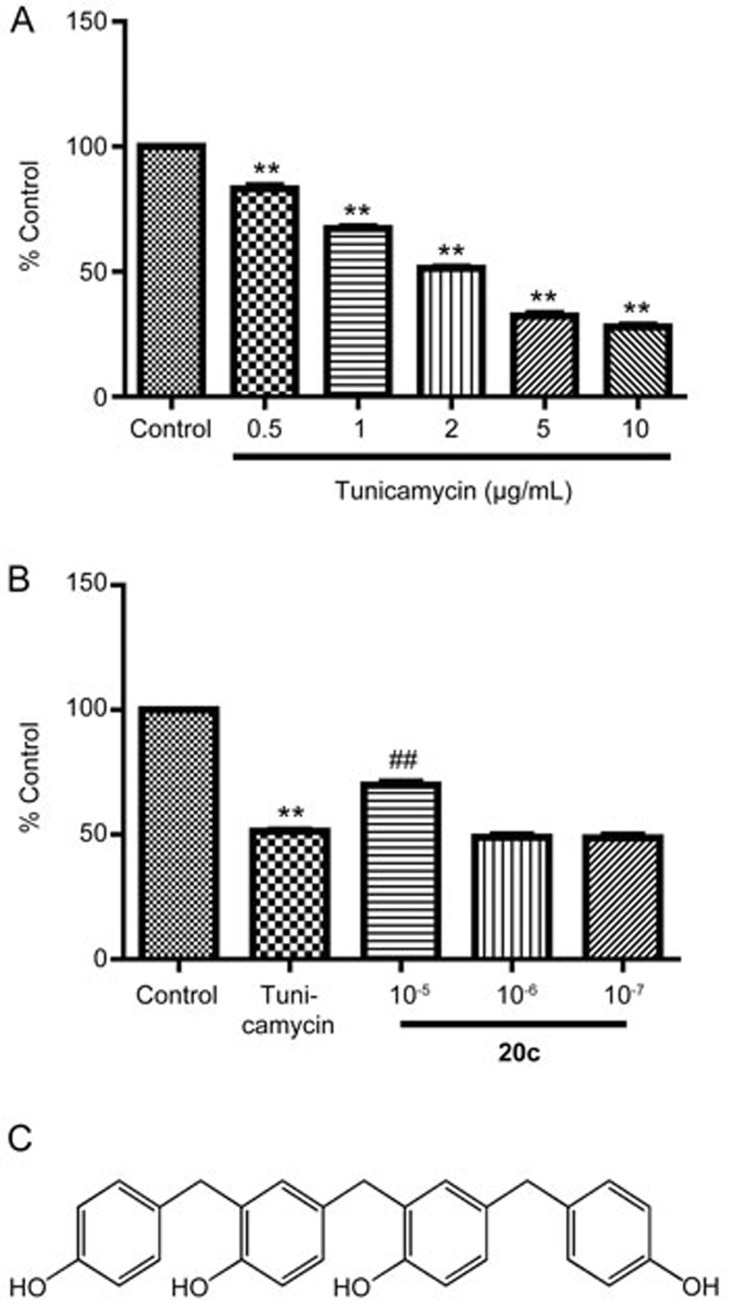
Compound 20c protected PC12 cells against the toxicity of tunicamycin. (A) PC12 cells were treated with tunicamycin at different concentrations (0.5, 1, 2, 5, and 10 μg/mL). Cell viability was analyzed with an MTT assay. The data are shown as the mean±SEM. n=6. One-way ANOVA with Dunnett's test was used for statistical analysis. **P<0.01 vs the control group. (B) After the PC12 cells were cultured for 24 h in 96-well plates, the cells were treated with tunicamycin 2 μg/mL or with tunicamycin 2 μg/mL and 20c (10−5, 10−6, and 10−7 mol/L). The data are shown as the mean±SEM. n=6. One-way ANOVA with Dunnett's test was used for statistical analysis. **P<0.01 vs the control group. ##P<0.01 vs the tunicamycin group. (C) The chemical structure of 20c.
In this study, our data suggest that tunicamycin, which is an ER stress inducer, increased the expression of the monomeric and oligomeric forms of α-syn and that these impacts were associated with the tunicamycin concentration and treatment time. Furthermore, the accumulation of two forms of α-syn in the ER and mitochondria was induced by tunicamycin in a time-dependent manner. 20c reduced the protein level of α-syn and inhibited ER stress by suppressing UPR activation. Together, ER stress increased the accumulation of the monomeric and oligomeric forms of α-syn, and 20c attenuated the damage induced by tunicamycin and promoted PC12 cell survival.
Materials and methods
Reagents
The compound 20c was obtained from the Department of Chemosynthesis, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). 20c was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 0.1 mol/L as a stock solution, which was stored at -80 °C until it was used.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide), tunicamycin, and DMSO were obtained from Sigma-Aldrich (St Louis, MO, USA). DMEM (Dulbecco's modified Eagle's medium), horse serum (ES) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). The following primary antibodies were used: anti-α-syn, anti-calnexin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-Grp78, anti-CHOP, anti-p-eIF2α, anti-eIF2α (1:1000, Cell Signaling Technology, Danvers, MA, USA); anti-ATF6 (1:500, Enzo Life Sciences, New York, NY, USA); anti-XBP1, anti-COX4 (1:1000, Abcam, Cambridge, UK); and anti-β-actin (1:5000, Sigma, St Louis, MO, USA). The secondary antibodies were purchased from KPL (1:5000, Gaithersburg, MD, USA).
Cell culture and treatment
Rat pheochromocytoma PC12 cells were maintained in our laboratory. The cells were cultured in DMEM containing 5% FBS and 5% ES and placed in a water-saturated atmosphere of 5% CO2 at 37 °C. The culture medium was changed every other day. PC12 cells were seeded at a density of 1×105 cells·cm−2.
Tunicamycin14 was dissolved in DMSO at a concentration of 10 mg/mL as a stock solution. The stock was stored at -80 °C until it was used. The PC12 cells were allowed to attach for 24 h before treatment. Then, the PC12 cells were treated with tunicamycin (0.5, 1, 2, 5, and 10 μg/mL) or treated with tunicamycin (2 μg/mL) and 20c (10−5, 10−6, and 10−7 mol/mL) for 24 h. Following the treatment, cell viability assessment, Western blot analysis, and immunofluorescence analysis were performed.
Assessment of cell viability
The cells were treated with tunicamycin or with tunicamycin and 20c for 24 h and cell viability was analyzed using an MTT assay15. The cells were seeded in 96-well plates at a density of 5×103 cells per well. After treatment for 24 h, 10 μL MTT (5 mg/mL) was added to each well and incubated at 37 °C for 4 h. Then, 100 μL SDS-HCl was added to each well, and the plate was incubated overnight at 37 °C. The optical density (OD) was analyzed at 570 nm on a Microplate Reader (Thermo Skanit Software 3.2, Germany)16.
Preparation of the subcellular fraction of the ER and mitochondria
PC12 cells were cultured in 10-cm plates at a density of 1×105 cells·cm−2 and were treated with tunicamycin (2 μg/mL) for 6, 12, or 24 h. The PC12 cells were collected at 4 °C. The subcellular fractions of the ER and mitochondria were extracted using the Tissue Endoplasmic reticulum Isolation Kit (Genmed Scientific Inc, Netherlands). The collected ER and mitochondria were used for Western blot analysis.
Immunofluorescence
Cells were seeded on slides coated with PLL for 24 h. After treatment with tunicamycin (2 μg/mL) for 12, 24, and 48 h, the cells were washed with ice-cold PBS three times for 5 min each time. The cells were fixed with 1% paraformaldehyde and then permeabilized with 0.1% Triton-X 100 for 10 min at room temperature. After washing the cells with PBS three times, the cells were blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich) for 30 min at room temperature and incubated with primary antibodies, including anti-CHOP or anti-α-syn (1:100), overnight at 4 °C. After washing the cells with PBS three times, the cells were incubated with an Alexa Fluor 488-conjugated secondary antibody, an Alexa Fluor 546-conjugated secondary antibody (1:500, Invitrogen, New York, USA) and Hoechst 33342 (1:1000, Life Technologies, NY, USA) for 1 h at room temperature. Images were obtained using a Leica inverted microscope equipped for fluorescence analysis (Leica Microsystems, Germany)17.
Western blot analysis
Following the treatments, the cultured medium was removed and cells were harvested in an ice bath. Total proteins were extracted as previously described17. PC12 cells were lysed in NP-40 lysis buffer (150 mmol/L NaCl, 1% Nonidet P-40, 50 mmol/L Tris, pH 7.4, and 1 mmol/L ethylenediamine tetraacetic acid) with a proteinase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). The cell lysates were centrifuged at 12 000×g for 20 min at 4 °C. The supernatant (soluble part) and the precipitant (insoluble part) were collected. The concentration of total protein for each sample was quantified with the Bicinchoninic Acid kit (Pierce, Rockford, IL, USA). Equal amounts of protein were loaded into the SDS-PAGE gels and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Boston, MA, USA). The membranes were blocked with 3% BSA and incubated with different primary antibodies at 4 °C overnight. The membranes were washed with TBST 3 times for 10 min each and incubated with secondary antibodies for 1 h at room temperature. Then, the membranes were washed with TBST three times, and the protein bands were detected with an enhanced chemiluminescence (ECL) plus detection system. The protein bands were analyzed using Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analyses
All data were analyzed using one-way analysis of Variance (ANOVA) with Dunnett's test. The data are shown as the mean±SEM. Differences with P<0.05 were considered statistically significant. All statistical analyses were performed using Prism 5 (GraphPad Software, Inc).
Results
Tunicamycin induced an increase in the monomeric and oligomeric forms of α-syn in PC12 cells
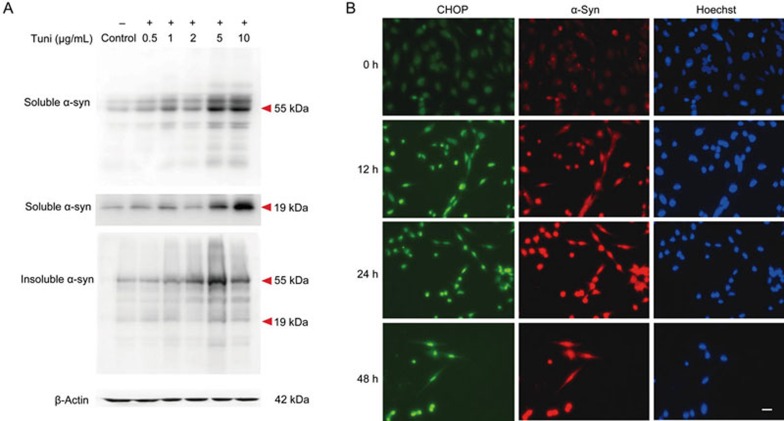
Studies have suggested that α-syn pathology can initiate ER stress in α-syn A53T mutant transgenic mice (αA53T tg mice) and that an inhibitor of ER stress, salubrinal, can significantly reduce the accumulation of α-syn in αA53T tg mice18. To determine whether an ER stress inducer could be directly associated with the accumulation of α-syn, we treated PC12 cells with tunicamycin at different concentrations (0.5, 1, 2, 5, and 10 μg/mL) for 24 h. The extracted protein was analyzed by Western blot analysis. The two bands, including one at approximately 19 kDa and another at 55 kDa, were the monomeric and oligomeric forms of α-syn. After tunicamycin administration, the protein levels of the monomeric and oligomeric forms of α-syn were increased in a dose-dependent manner in both the soluble and insoluble parts of the protein extracted (Figure 1A). Subsequently, we treated the PC12 cells with tunicamycin (2 μg/mL) for 12, 24, and 48 h. The protein level of CHOP increased with the treatment time. Meanwhile, the protein expression of α-syn increased in a time-dependent manner and was co-located with the increase in CHOP (Figure 1B). These data suggest that tunicamycin led to the accumulation of the monomeric and oligomeric forms of α-syn and that this accumulation was associated with the concentration and duration of tunicamycin treatment.
Figure 1.
Tunicamycin induced accumulation of the α-syn monomer and oligomer. (A) PC12 cells were treated with tunicamycin at different concentrations (0.5, 1, 2, 5, and 10 μg/mL) for 24 h. The total protein was collected and analyzed using Western blot analysis. The soluble part of the protein fraction was the supernatant collected after centrifugation. The insoluble part of the protein fraction was the precipitant collected after centrifugation, which included the insoluble aggregates of α-syn. Respective immunoblots of α-syn are shown. β-Actin was the loading control. (B) PC12 cells treated with tunicamycin for 12, 24, and 48 h were immunostained with antibodies against CHOP (green) and α-syn (red). Nuclei were detected with Hoechst 33342 (blue). Scale bar, 100 μm.
Accumulation of α-syn induced by tunicamycin occurred in the ER and mitochondria
Because the accumulation of α-syn was induced by tunicamycin, we identified the disturbance of α-syn in the ER after treating the cells with tunicamycin. First, we extracted the microsome (ER) and mitochondria fractions of PC12 cells (Figure 2A) and analyzed the alteration of α-syn using Western blot analysis. Surprisingly, the protein levels of the monomeric form of α-syn in the ER increased in a time-dependent manner after tunicamycin treatment. After 24 h, the oligomeric form of α-syn in the ER increased as well. Additionally, the accumulation of the α-syn monomer and oligomer in mitochondria increased after tunicamycin treatment. After 24 h, the oligomeric form of α-syn had a similar increase in the mitochondria as it did in the ER (Figure 2B). All of the data suggest that the monomeric and oligomeric forms of α-syn were enriched in a time-dependent manner in the ER and mitochondria fractions after tunicamycin administration.
Figure 2.
Tunicamycin led to the accumulation of α-syn in the ER and mitochondria, and the accumulation increased with treatment time. (A) Protocol for the extraction of ER and mitochondria. (B) The expression of α-syn accumulated in the ER and mitochondria. Calnexin and COX4 were organelle-specific markers: calnexin for the ER and COX4 for the mitochondria. Respective immunoblots of α-syn in the ER and mitochondria are shown.
20c significantly attenuated the accumulation of α-syn induced by tunicamycin
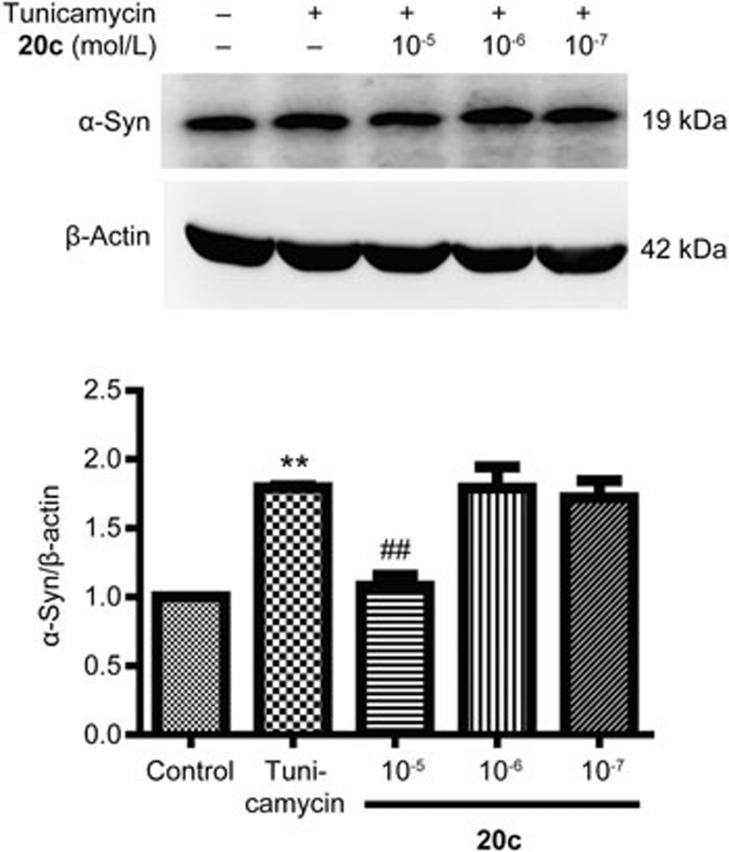
The major component of LBs is α-syn. Numerous data suggest that increased levels of α-syn lead to neurodegeneration. New compounds to ameliorate or prevent the α-synucleinopathies have attracted a great deal of attention19. First, we treated PC12 cells with tunicamycin at different concentrations (0.5, 1, 2, 5, and 10 μg/mL) and analyzed cell viability using an MTT assay. Compared to the control group, cell viability decreased according to the concentration of tunicamycin. When the PC12 cells were treated with tunicamycin (2 μg/mL), the cell viability declined to 51.29 % (Figure 3A). Therefore, 2 μg/mL was used in subsequent tests. Previous data indicated that 20c protected PC12 cells against the neurotoxicity of rotenone. To determine whether 20c attenuated the damage induced by tunicamycin, we examined the effects of 20c on cell viability after treating the cells with tunicamycin. 20c (10−5 mol/L) significantly enhanced the cell viability to 69.85% (% control group), which suggested that 20c could protect PC12 cells against the damage induced by tunicamycin (Figure 3B). Furthermore, to determine whether 20c attenuated the accumulation of α-syn induced by tunicamycin, we treated the PC12 cells with tunicamycin or a combination of tunicamycin and 20c. Compared with the tunicamycin group, 20c (10−5 mol/L) notably decreased the protein level of α-syn (Figure 4). These data suggest that 20c protected the PC12 cells against insults and decreased the protein level of α-syn induced by tunicamycin.
Figure 4.
Compound 20c attenuated the increase of α-syn expression induced by tunicamycin (2 μg/mL). Respective immunoblots and statistical analysis of the protein level of α-syn are shown. The data are shown as the mean±SEM. n=4–5. One-way ANOVA with Dunnett's test was used for statistical analysis. **P<0.01 vs the control group. ##P<0.01 vs the tunicamycin group.
20c inhibited ER stress by suppressing the UPR
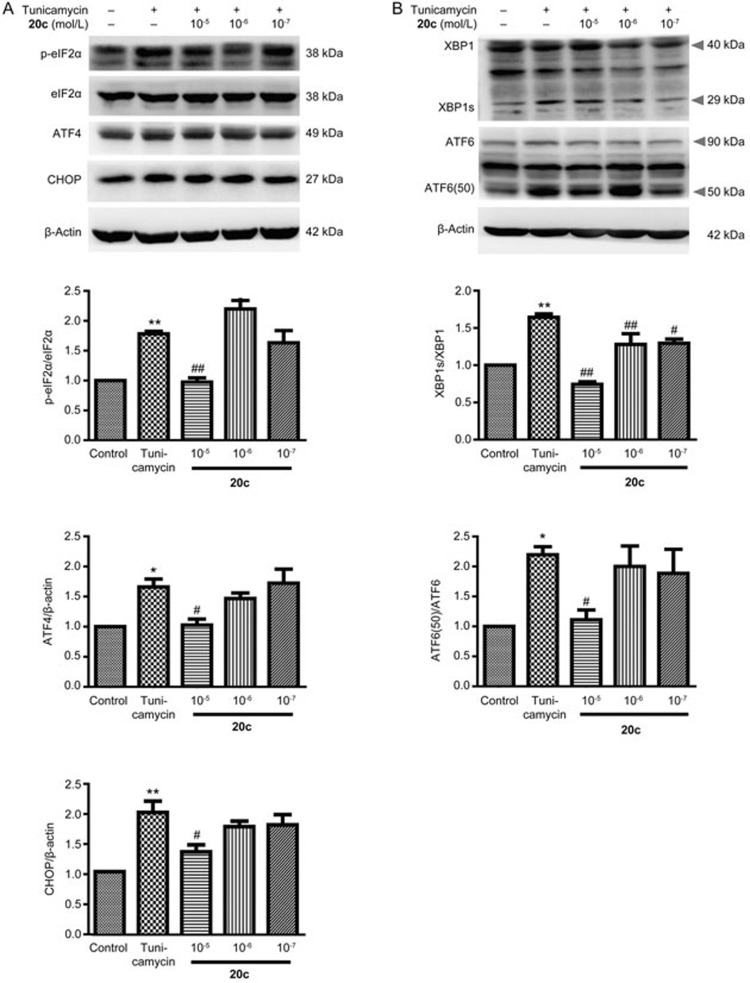
The ER is the central intracellular organelle that is responsible for the synthesis, quality control, and degradation of proteins. Because 20c attenuated the increase of α-syn, we speculated that 20c inhibited the activation of ER stress. After treating the PC12 cells with tunicamycin or with tunicamycin and 20c, we analyzed the protein level of Grp78 (a marker of ER stress). Compared with the control group, tunicamycin significantly increased the protein level of Grp78. We found that 20c (10−5 mol/L) remarkably decreased the expression of Grp78 (Figure 5). To determine the effects of 20c on the activation of UPR, we analyzed the protein levels of ER-stress-associated downstream chaperones. Compared to the control group, the phosphorylation of eIF2α and the expression of transcription factor ATF4 were significantly increased by tunicamycin, which meant that the PERK signaling pathway was activated. We also determined that 20c markedly reduced the phosphorylation of eIF2α and the expression of ATF4 and that 20c inhibited the PERK pathway (Figure 6A). Similar to the effects of 20c on the PERK pathway, the activation of the IRE1 and the ATF6 pathways was notably inhibited by 20c and was accompanied by a decrease in the protein levels of XBP1 and ATF6 (50) (Figure 6B). These data suggest that 20c suppressed the activation of the major branches of the UPR, which suggests that 20c can inhibit ER stress.
Figure 5.
Compound 20c inhibited the activation of ER stress. Respective immunoblots and statistical analysis of the protein level of Grp78 are shown. The data are shown as the mean±SEM. n=4–5. One-way ANOVA with Dunnett's test was used for statistical analysis. **P<0.01 vs the control group. #P<0.05 vs the tunicamycin group.
Figure 6.
Compound 20c suppressed the initiation of UPR. (A, B) Respective immunoblots and statistical analysis of the protein levels of p-eIF2α, eIF2α, ATF4, CHOP, XBP1s/XBP1, and ATF6(50)/ATF6. Data are shown as the mean±SEM. n=4–5. One-way ANOVA with Dunnett's test was used for statistical analysis. *P<0.05, **P<0.01 vs the control group. #P<0.05, ##P<0.01 vs the tunicamycin group.
Discussion
The presence of potentially pathogenic protein aggregates is a common characteristic shared by PD and other PMDs20. However, whether ER stress directly contributes to the accumulation or aggregation of α-syn has not been clarified. Our data indicate that the accumulation of α-syn is directly induced by an ER stress inducer and that the increase of the monomeric or oligomeric forms of α-syn is associated with the tunicamycin concentration and treatment time. Additionally, the two forms of α-syn accumulated in the ER and mitochondria after treatment with tunicamycin, which suggests that ER stress was an active participant in the process of α-syn accumulation. Moreover, our studies provide evidence that 20c exerts protective effects on tunicamycin-treated PC12 cells, reduces the expression of α-syn and inhibits the activation of the UPR, which suggest that 20c is a neuroprotective compound worthy of future study.
The aggregation of α-syn leads to lysosomal dysfunction21, membrane disruption22, and dopaminergic neuron disorders20. Numerous studies have proposed that the aggregation of α-syn or the increased Ser129 phosphorylation of α-syn are co-localized with UPR activation23,24. ER stress initiates the UPR to modulate cellular response to restore ER homeostasis. The UPR leads to the simultaneous activation of adaptive and pro-apoptotic responses25. Under chronic ER stress, when the ER homeostasis fails to be recovered, the UPR triggers cell death through apoptosis3. The ER-stress-associated apoptosis pathway is the central pathway for eliminating damaged cells. The best characteristic of the pro-apoptotic pathway is the production of the CHOP transcription factor, which is modulated by ATF4, and possibly by ATF6 and XBP126,27. In our studies, tunicamycin increased the expression of α-syn in a time-dependent manner. Accompanied by enhancement of the ER-stress intensity, the viability of PC12 cells was reduced. That decrease in viability is the reason why, after 48 h of tunicamycin treatment, the number of cells in the image was less than other groups. On the other hand, 20c attenuated the decrease in cell viability induced by tunicamycin, which indicated that 20c could protect against ER-stress-associated insults. Caspase-12-caspase-3 is another ER-stress-associated apoptosis pathway. Caspase-12 can be specifically cleaved and activated after the activation of the IRE1 pathway28. The effects of 20c on the activation of caspase-12 have yet to be clarified.
The accumulation of α-syn in the ER due to tunicamycin treatment is consistent with recent studies suggesting that α-syn accumulates in the ER as PD progresses1. The level of α-syn in the ER of presymptomatic αA53T tg mice was proportional to the total α-syn. Subsequently, higher molecular weight α-syn was enriched in the ER in symptomatic tg mice. The accumulation also occurred selectively in pathologically affected brain fields, such as SNpc, which suggests that the accumulation of α-syn in the ER is a primary characteristic of PD. The accumulation of α-syn could trigger ER stress by interacting with the ER chaperone Grp78 or by disturbing Ca2+ metabolism29,30. Moreover, the overexpression of α-syn in yeast blocked ER-Golgi vesicular trafficking, which contributed to toxicity, cell loss and selective impacts on dopamine-producing neurons19. Furthermore, α-syn disrupts UPR by interacting with ATF6 and blocking the incorporation of ATF6 into COPII vesicles31. These studies indicate that the accumulation of α-syn may trigger the activation of ER stress. Furthermore, the accumulation of α-syn in the mitochondria was induced after treatment with tunicamycin. Bir et al observed that the intracellular accumulation of α-syn in SHSY5Y cells led to mitochondrial impairment and cell death through interaction with the permeability transition pore complex in isolated preparations32. Moreover, monomers and oligomers of α-syn A53T localized to the mitochondrial membranes, and this localization was associated with selective age-related mitochondrial complex I inhibition, which suggested that mitochondrial α-syn affects cells and led to mitochondria dysfunction33. The impact of 20c on the expression of α-syn in the ER and mitochondria should be further studied.
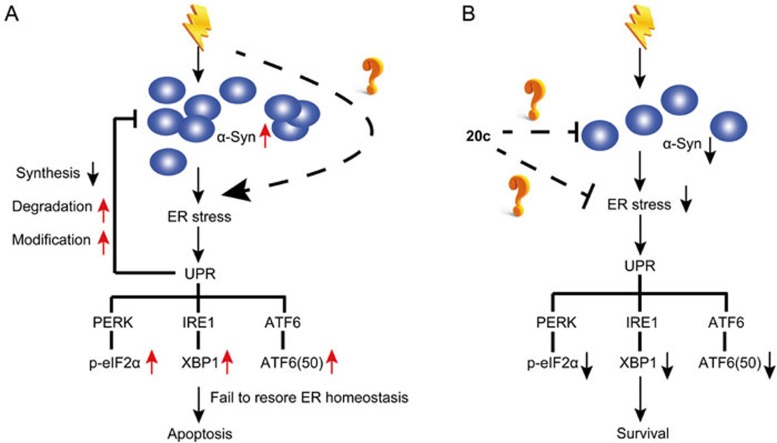
Based on the current studies and our data supporting a relationship between ER-stress-associated toxicity and the accumulation of α-syn, we propose a model (Figure 7A). Normally, α-syn is located in the lumen of ER. Upon stimulation by tunicamycin or other stimuli, the monomeric form of α-syn increases and aggregates into the oligomeric form of α-syn. Finally, the oligomeric form matures into insoluble aggregates as the disease progresses. The accumulation or aggregation of α-syn triggers the activation of ER stress, and chronic ER stress leads to the initiation of ER-stress-associated apoptosis. As feedback, ER dysfunction exacerbates the increase in α-syn expression. Given the therapeutic effects of salubrinal on reducing α-syn oligomers in the ER1, the abnormal accumulation of α-syn contributes to chronic ER stress and ER-stress-associated insults.
Figure 7.
A model showing the protective effects of 20c on tunicamycin-treated cells based on our data. (A) Accumulation of α-syn triggers the activation of ER stress. ER stress initiates the UPR to restore ER homeostasis. When UPR fails to recover ER homeostasis, ER-stress-specific apoptosis is promoted to eliminate the damaged cell. (B) Compound 20c may inhibit the accumulation of α-syn, which suppresses the activation of ER stress. Finally, 20c inhibits the activation of PERK, IRE1, and ATF6 signaling pathways, which promotes cell survival. However, the mechanism through which 20c reduces the accumulation of α-syn has yet to be clarified.
Current pharmacological therapies for PD only attenuate the symptoms and fail to prevent the progression of PD15. Therefore, new therapeutic compounds should be very attractive to researchers. Previous studies in our laboratory provide evidence that 20c protected against neurotoxicity in H2O2-and rotenone-treated models. Based on the model that we have proposed, 20c attenuated the accumulation of α-syn, which alleviated the activation of UPR by suppressing all three central downstream pathways. As a result, 20c improved cell viability, which was decreased by tunicamycin (Figure 7B). However, the mechanism through which 20c decreased the expression of α-syn has yet to be determined. Numerous pathological conditions trigger ER stress and result in cell death, including disturbance of ER Ca2+ homeostasis, ER-Golgi vesicular trafficking, and local oxidative stress5,34. Compound 20c was observed to be an anti-oxidative compound. The next step would be to identify the mechanism of protection that 20c exerts against the toxicity induced by tunicamycin and to determine whether 20c attenuates the accumulation and inhibits the initiation of UPR through anti-oxidative effects and modulation of ER Ca2+ homeostasis.
Together, the data obtained in our studies suggest that tunicamycin treatment led to neurotoxicity, decreased cell viability, and increased the accumulation of the monomeric and oligomeric forms of α-syn. Additionally, tunicamycin promoted the accumulation of two forms of α-syn in the ER and mitochondria in a time-dependent manner. Furthermore, 20c reduced the accumulation of α-syn and inhibited the initiation of UPR. The data indicate that modulation of ER stress is involved in the neuroprotective action of 20c. We therefore hope that 20c will be a compound that leads to new PD therapies and that regulating ER stress will provide a new theoretical basis for additional studies.
Author contribution
Zheng MOU and Nai-hong CHEN designed the research study; Zheng MOU performed experiments and data analysis; Yu-he YUAN, Yu-xia LOU, Ju-yang HUANG, Cong-yuan XIA, Yan GAO, Shi-feng CHU, Piao LUO, and Yang HENG helped prepare the paper; Cheng-gen ZHU and Jian-gong SHI contributed to the preparation of 20c.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81274122, 81373997, 81273629, 81473376, U1402221, and 81573640), the National Mega-project for Innovative Drugs (No 2012ZX09301002-004), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (No IRT1007), Beijing Natural Science Foundation (No 7131013 and 7161011), and the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No BZ0150).
References
- Jiang P, Gan M, Ebrahim AS, Lin WL, Melrose HL, Yen SH. ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener 2010; 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiss CC, Braun TS, Konings IB, Grabmayr H, Hassink GC, Sidhu A, et al. Functionally different alpha-synuclein inclusions yield insight into Parkinson's disease pathology. Sci Rep 2016; 6:23116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G, Valdes P, Hetz C. An ERcentric view of Parkinson's disease. Trends Mol Med 2013; 19: 165–75. [DOI] [PubMed] [Google Scholar]
- Soto C. Transmissible proteins: expanding the prion heresy. Cell 2012; 149: 968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 2004; 14: 20–8. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008; 7: 1013–30. [DOI] [PubMed] [Google Scholar]
- Halliday M, Mallucci GR. Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology 2014; 76: 169–74. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 2013; 12: 105–18. [DOI] [PubMed] [Google Scholar]
- Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal 2015; 8: ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 2008; 115: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CF, Ko CH, Koon CM, Chin WC, Themis Kwong HC, Lo AW, et al. The aqueous extract of rhizome of Gastrodia elata Blume attenuates locomotor defect and inflammation after traumatic brain injury in rats. J Ethnopharmacol 2016; 185: 87–95. [DOI] [PubMed] [Google Scholar]
- Manavalan A, Ramachandran U, Sundaramurthi H, Mishra M, Sze SK, Hu JM, et al. Gastrodia elata Blume (tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol 2012; 3: 219–41. [PMC free article] [PubMed] [Google Scholar]
- Ramachandran U, Manavalan A, Sundaramurthi H, Sze SK, Feng ZW, Hu JM, et al. Tianma modulates proteins with various neuro-regenerative modalities in differentiated human neuronal SH-SY5Y cells. Neurochem Int 2012; 60: 827–36. [DOI] [PubMed] [Google Scholar]
- Kogel D, Schomburg R, Schurmann T, Reimertz C, Konig HG, Poppe M, et al. The amyloid precursor protein protects PC12 cells against endoplasmic reticulum stress-induced apoptosis. J Neurochem 2003; 87: 248–56. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zu G, Zhang X, Wang X, Li S, Gong X, et al. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology 2016; 101: 480–9. [DOI] [PubMed] [Google Scholar]
- Dong G, Chen T, Ren X, Zhang Z, Huang W, Liu L, et al. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016; 26: 7–18. [DOI] [PubMed] [Google Scholar]
- Ma KL, Song LK, Yuan YH, Zhang Y, Han N, Gao K, et al. The nuclear accumulation of alpha-synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology 2014; 82: 132–42. [DOI] [PubMed] [Google Scholar]
- Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, et al. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci 2012; 32: 3306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 2006; 313: 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao XQ, Wang XL, Zhang D. FLZ attenuates alpha-synuclein-induced neurotoxicity by activating heat shock protein 70. Mol Neurobiol 2016. DOI: 10.1007/s12035-015-9572-9. [DOI] [PubMed]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A 2016; 113: 1931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Sharikov Y, Kouznetsova VL, Greenberg JP, Wrasidlo W, Overk C, et al. Molecular determinants of alpha-synuclein mutants' oligomerization and membrane interactions. ACS Chem Neurosci 2015; 6: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, et al. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem 2008; 283: 23179–88. [DOI] [PubMed] [Google Scholar]
- Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 2005; 14: 3801–11. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 2006; 4: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 2002; 318: 1351–65. [DOI] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 2002; 366: 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ 2006; 13: 385–92. [DOI] [PubMed] [Google Scholar]
- Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M, et al. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron 2010; 68: 865–78. [DOI] [PubMed] [Google Scholar]
- Belal C, Ameli NJ, El Kommos A, Bezalel S, Al'Khafaji AM, Mughal MR, et al. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant alpha-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum Mol Genet 2012; 21: 963–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle JJ, Forcelli PA, Delannoy M, Oaks AW, Permaul E, Berry DL, et al. alpha-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson's disease. Neurobiol Dis 2015; 76: 112–25. [DOI] [PubMed] [Google Scholar]
- Bir A, Sen O, Anand S, Khemka VK, Banerjee P, Cappai R, et al. alpha-Synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: implications in the pathogenesis of Parkinson's disease. J Neurochem 2014; 131: 868–77. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 2010; 486: 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 2014; 15: 233–49. [DOI] [PubMed] [Google Scholar]