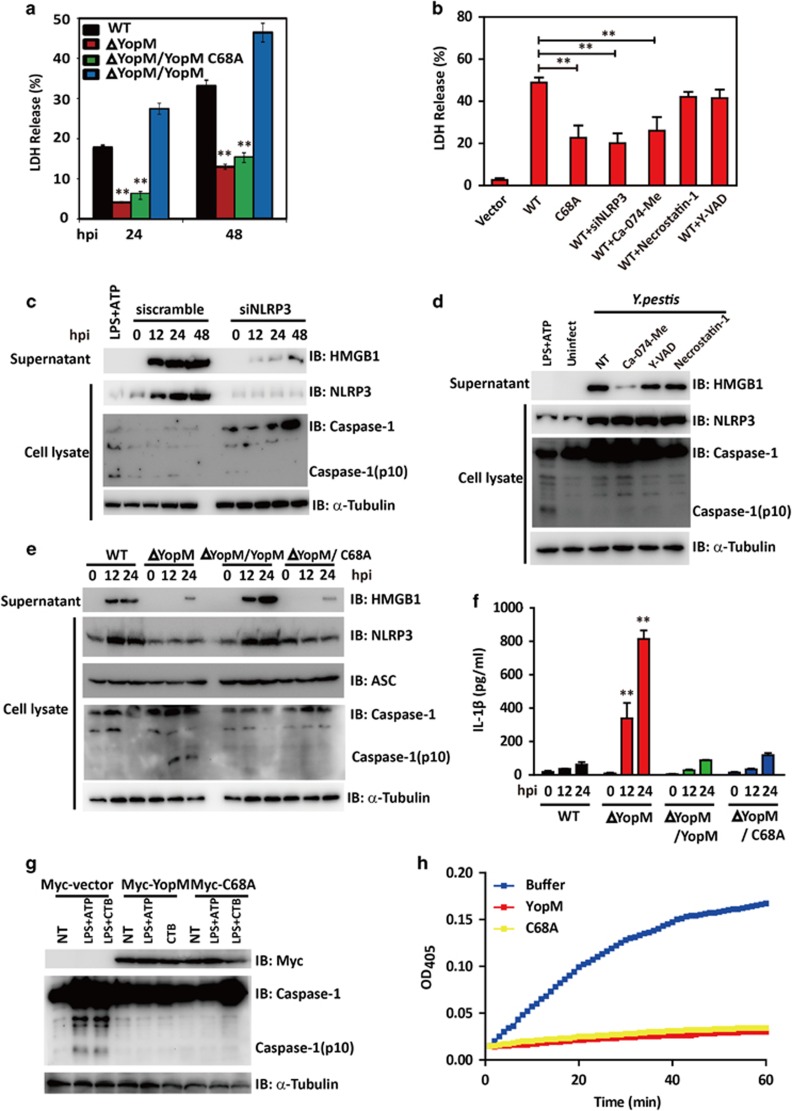
Figure 5.
The importance of YopM E3 ligase activity in NLRP3-mediated necrotic cell death. (a) BMDMs were infected, respectively, with Y. pestis WT, ΔYopM, ΔYopM/YopM or ΔYopM/YopM C68A mutant bacteria for the indicated time (MOI=20). After Y. pestis invasion, supernatants from macrophages were collected at the time points indicated, and percent LDH was determined. (b) BMDMs cells were treated with or without 100 μM caspase-1-specific inhibitor YVAD-CHO, 50 μM Cathepsin B-specific inhibitor Ca-074-Me or 30 μM RIP1-specific inhibitor necrostatin-1 and then transfected with expression plasmids encoding for Flag-YopM or its mutants or NRLP3 siRNA oligos (siNRLP3). Supernatants were collected at 24 h after transfection and analyzed for LDH release. (c) BMDMs cells were transfected with scrambled or NLRP3 siRNA oligos (si NLRP3) and then infected with Y. pestis. Cell extracts and supernatants were prepared at indicated time points. The whole-cell lysates were analyzed by immunoblotting with anti-NLRP3, anti-caspase-1 Abs. The supernatants were analyzed by immunoblotting with anti-HMGB1. α-Tubulin was used as equal loading control. (d) BMDMs cells were treated with or without 100 μM caspase-1-specific inhibitor YVAD-CHO, 50 μM Cathepsin B-specific inhibitor Ca-074-Me or 30 μM RIP1-specific inhibitor necrostatin-1 before infection. BMDMs were infected with Y. pestis, cell extracts and supernatants were prepared at 24 h. The whole-cell lysates were analyzed by immunoblotting with anti-NLRP3, anti-caspase-1 Abs. The supernatants were analyzed by immunoblotting with anti-HMGB1. α-Tubulin was used as equal loading control. (e and f) BMDMs were infected, respectively, with Y. pestis WT, ΔYopM, ΔYopM/YopM, or ΔYopM/YopM C68A mutant bacteria for the indicated time (MOI=20). After Y. pestis invasion, cell extracts and supernatants were prepared at indicated time points. The whole-cell lysates were analyzed by immunoblotting with anti-NLRP3, anti-ASC, and anti-caspase-1 Abs. The supernatants were analyzed by immunoblotting with anti-HMGB1 Abs (e) or analyzed by ELISA for IL-1β (f).α-Tubulin was used as equal loading control. (g) BMDMs cells were transfected with the expression vector encoding Myc-YopM, Myc-YopM C68A. After 24 h, cells were primed for 4 h with 1 ng/ml LPS and stimulated with ATP (2.5 mM) for 30 min or CTB (20 mg/ml) for 16 h. The whole-cell lysates were analyzed by immunoblotting with anti-Myc and anti-caspase-1 Abs. α-Tubulin was used as equal loading control. (h) Recombinant caspase-1 was preincubated with GST-YopM or GST-YopM(C68A) for 5 min at 37 °C in assay buffer. Caspase-1 substrate Ac-YVAD-pNA was added, and cleavage was detected by monitoring of absorbance at 405 nm. Cell-based studies were performed at least three times independently with comparable results. Data were presented as mean±S.E.M. Student's t-test was used for statistical analysis: **P<0.01