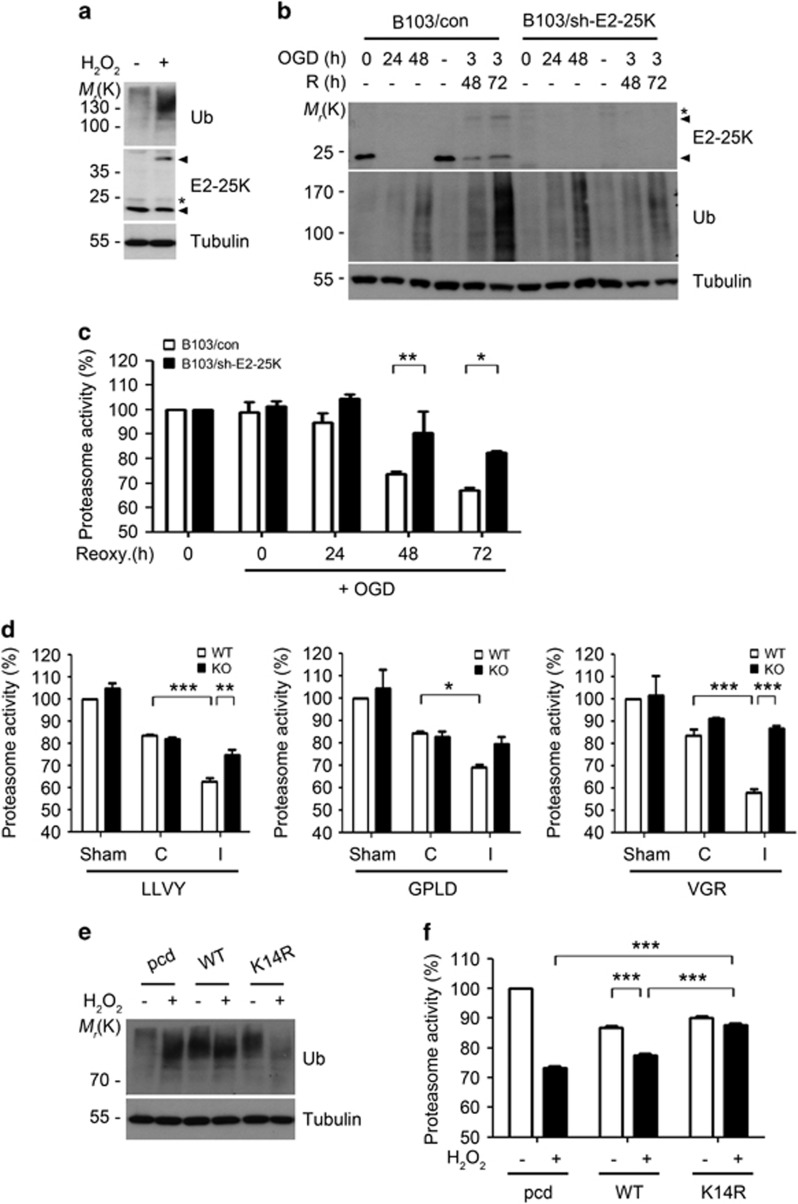
Figure 4.
E2-25K SUMOylation under I/R condition impairs proteasome activity. (a and b) SH-SY5Y cells were treated with 100 μM H2O2 for 12 h (a) and B103/con and B103/sh-E2-25K cells were exposed to OGD alone or followed by reoxygenation for the indicated times (b). Cell extracts were analyzed by western blotting. (c) B103/con and B103/sh-E2-25K cells were exposed to OGD for 3 h and reoxygenation for the indicated times. Cell lysates were analyzed for the proteasome activity using suc-LLVY-AMC. Values represent mean±S.E.M. (n=3, two-way ANOVA followed by Bonferroni's post hoc test, *P<0.05, **P<0.01). (d) E2-25K WT and KO mice were perfused for 24 h after MCAO for 30 min. Tissue lysates (except cerebellum) from non-ischemic (contralateral, C) and ischemic (ipsilateral, I) hemispheres of mouse brains were examined for chymotrypsin-like (Suc-LLVY-AMC), caspase-like (Ac-GPLD-AMC) and trypsin-like (Bz-VGR-AMC) activities of the proteasome (mean±S.E.M., sham WT and KO mice, n=3; MCAO WT mice, n=3; KO mice, n=4, two-way ANOVA followed by Bonferroni's post hoc test, *P<0.05 **P<0.01, ***P<0.001). (e and f) SH-SY5Y cells were transfected with pcDNA (pcd), E2-25K WT or K14R, treated with 100 μM H2O2 for 12–20 h and analyzed by western blotting (e) or examined for proteasome activity using suc-LLVY-AMC (f). Bars represent mean±S.E.M. (n=3, two-way ANOVA followed by Bonferroni's post hoc test, ***P<0.001)