Table 1.
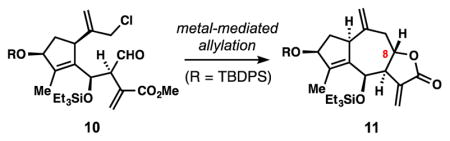
Investigation of metal-mediated allylation conditions for the synthesis of the 5,7,5-fused guaianolide lactone system.
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Conditions[a,b] | 11 | 14 | 15 | rsm |
| 1 | CrCl2, cat. NiCl2, DMF, 60 °C | 10%[e] | 17% | ↕ | ↕ |
| 2 | In0, NaI, DMF, 60 °C | 13%[f] | ↕ | ↕ | ↕ |
| 3[c] | NaI; SmI2, HMPA-THF, −78 °C | 27%[e] | 17% | ↕ | ↕ |
| 4[c] | NaI; Zn0, aq. NH4Cl, THF, rt | 0% | 51% | ↕ | 34% |
| 5[c] | NaI; Mg0, cat. (CH2Br)2, THF, rt | 0% | ↕ | ↕ | ↕ |
| 6[c] | NaI; iPrMgCl, THF, 0 °C | 0% | ↕ | ↕ | ↕ |
| 7[c] | NaI; SnCl2, DMF, rt | 53%[f] | ↕ | 20% | 9% |
| 8[d] | SnCl2, NaI, DMF, 60 °C | 90%[f] | ↕ | ↕ | ↕ |
Reaction performed on a 30-mg scale unless otherwise stated;
Isolated yields are reported;
Starting material first reacted with NaI in acetone for 8 h;
Reaction performed on a 7-gram scale;
Diastereomeric ratio 2:1 11:8-epi-11;
Single diastereomer obtained.
