Abstract
Introduction: The purpose of the present study was to assess the clinical effects of anti-microbial photodynamic therapy (PDT) after closed surface scaling in the treatment of peri-implant diseases.
Methods: Ten patients with a total of 15 pairs of dental implants, showing clinical and radiographic signs of peri-implant diseases, were included in this study. In each patient, one implant randomly served as control implant and the other served as test implant. The control implants were treated with closed surface scaling only and the test implants received additionally PDT, using light with a wavelength of 630 nm and intensity of 2000 mw/cm2 for 120 seconds after application of photosensitizer in peri-implant sulcus. Clinical parameters were evaluated before and 1.5 and 3 months after treatment.
Results: Statistical analysis showed significant differences in probing pocket depth (PPD), clinical attachment loss (CAL), bleeding on probing (BOP), and gingival index (GI) at each time point between the two groups. There were no statistically significant changes with respect to any of the parameters in the control group. Complete resolution of BOP at 3 months was achieved in 100% of test implants. At 1.5 and 3 months, there were significant differences in the mean probing depth and CAL gain measurements at implants in the test group.
Conclusion: The present study revealed that adjunctive use of PDT following closed surface scaling could lead to clinical improvement of peri-implant diseases. Further studies are necessary to confirm our results.
Keywords: clinical trial, dental implant, mechanical debridement, peri-implantitis, photodynamic therapy
Introduction
Replacement of lost tooth with dental implants was such an experience for patients, as they have their own teeth for the third time. Nowadays, dental implants are considered to be one of the most commonly used treatment options in the replacement of missing teeth.1 Because of frequent use of dental implants, peri-implant diseases have become an increasing problem in recent years.2 The term “peri-implant diseases” is collectively used for inflammatory lesions that may affect just the peri-implant mucosa which is named peri-implant mucositis or may also result in loss of supporting bone, so the condition is termed peri-implantitis.3 Peri-implant diseases if not successfully treated, may progress to complete loss of osseointegration and so implant.4
Because microbial colonization plays an important etiological role in disease development, it was assumed that removal of bacterial biofilm from the implant surface is a prerequisite for the treatment.5,6 So the primary goal of therapy is to control disease progression by decontaminating infected implant surfaces.7 Although the final goal of the therapy is reosseointegration and bone regeneration around implants, previous studies have demonstrated that reosseointegration around diseased implants is very difficult to achieve.8
A broad variety of different antimicrobial treatment modalities such as surgical and non-surgical approaches, chemical and mechanical decontamination, laser treatment or therapies with antiseptics or antibiotics have been proposed to achieve this goal.9-13 Unfortunately, prevention of bacterial colonization on the micro structured dental implant surface seems to be impossible. Bacterial colonization occurs rapidly on oral implant surfaces, after installation in oral cavity.14 However, decontamination of rough implant surfaces is very difficult.15 Moreover, some of these treatment modalities led to undesirable outcomes such as implant surface alteration, bacterial resistance.16,17 So, novel approaches are still necessary to solve this problem.
Photodynamic therapy (PDT) is a simple, non-invasive technique that proved to have anti-bacterial effects according to the findings of previous authors.18,19 So it could be suggested that this method can be successfully used in the treatment of pathological conditions with bacterial etiology such as peri-implant diseases and periodontal diseases.20,21 PDT uses a low-level laser, following the application of photosensitizing substances such as TBO.22 It is believed that PDT may lead to changes in plasma membrane and cause DNA damages by singlet oxygen.23 Earlier studies illustrate that antimicrobial PDT has been effective in reducing the prevalence of pathogens on implant surfaces without any side effects on implants or surrounding bone and tissues.24,25 However, there are very limited clinical studies and clinical effects of PDT have not yet been demonstrated. To the best of our knowledge, until now no investigation from randomized controlled clinical studies are available that determine the clinical effects of PDT in the treatment of peri-implant diseases. The aim of this study was to evaluate the clinical efficacy of PDT application after closed surface scaling in the treatment of peri-implant diseases.
Methods
Study Design
The study was performed as a randomized, split-mouth clinical trial (IRCT201309119260N2, http://www.irct.ir/). This study was conducted from March 2011 up to July 2012. Dental implants with diagnosis of peri-implant diseases (peri-implant mucositis or peri-implantitis) were identified at an initial clinical and radiographic examination. All patients were informed of procedures, purpose, duration, and outlines of the study and signed a written informed consent form, prior to the study. The ethics committee of Islamic Azad University, Tehran, Iran, approved the study (22008), which was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. The sample size was of at least 10 implants in each group, making a total of 20 implants, and resulted in a power of 90% to detect a mean difference of 1.1 mm in CAL between groups.
Study Population
The study population consisted of 30 implants in 10 partially edentulous patients (2 men and 8 women, with a mean age of 52.8 years), each of whom displayed at least two screw type dental implants presenting peri-implant diseases placed in different quadrants. The study population is summarized in Table 1.
Table 1 . Baseline Demographics of Patients/Implants .
| Parameters | Total Number (n = 10) |
| Women | 8 |
| Men | 2 |
| Mean age of patients ( mean± SD) | 52.8 ± 7.33 |
| Total number of implants | 30 |
| Smokers | 2 |
| Diabetes | 2 |
| History of periodontitis | 5 |
Among the included implants, 20 of them (in 10 patients) exhibited peri-implantitis and 10 implants (in 5 patients) were diagnosed as peri-implant mucositis. Implants supported either cemented fixed prosthesis or removable prosthesis. There were dental implants with 3 different surface characteristics: SLA surface (Dentium, Implantium, South Korea) (n=26), RBM surface (BIDC, Biohorizon, USA) (n=2), V-TPS surface (Innova, Oraltronics, Germany) (n=2).
In each patient, included dental implants of different quadrants were randomly divided into 2 groups of equal size (n=15), according to the therapy.
Control group: consisted of 15 dental implants, received only closed surface scaling.
PDT group: consisted of 15 dental implants, were followed by PDT after closed surface scaling.
Randomization
Randomization was performed for each patient separately; using coin toss and dental implants of the same disease were assigned to the control and PDT groups. The randomization process led to comparable mean values of all investigated clinical parameters at baseline in all groups.
Patient Selection Criteria
For patient selection, the following inclusion criteria were defined:
Presence of at least two screw type titanium dental implants, in different sites, exhibiting clinical and radiographic signs of peri-implant diseases (including peri-implant mucositis and peri-implantitis).
No implant mobility
No evidence of occlusal overload
No treatment of peri-implant diseases for at least 6 months before the study
No use of antibiotics and anti-inflammatory drugs for the 3 months prior to the treatment. If these drugs were taken during the study, patient would be excluded.
At least 1 year function of implants.
No pregnancy and nursing
No uncontrolled diabetes (HbA1c < 7)
In cases where subjects had a history of periodontitis, they were included if the lesions were treated at the remaining teeth and diseases were halted.
Patients were examined clinically and radiographically by an experienced examiner and the implants diagnosed as healthy implants, peri-implant mucositis, or peri-implantitis. Dental implants with diagnosis of peri-implant mucositis or peri-implantitis were included in this study. The inclusion criteria for peri-implant mucositis were:
Presence of bleeding on probing (BOP)
Soft tissue redness
Probing pocket depth (PPD) < 5 mm
No peri-implant bone loss in radiographs
Dental implants with peri-implantitis presented horizontal, vertical, or saucer shape peri-implant bone loss in parallel periapical radiographs, compared with control x-ray obtained after prosthesis delivery, in addition to exhibiting at least 2 of the following clinical signs:
BOP
Suppuration and fistula
Mucosal swelling and redness
PPD > 5 mm
Mucosal recession
The demographic and systemic profiles of the individuals were assessed via questionnaires and the history of periodontitis, time of implant loading, number of implants, and surgical procedures were obtained from documented patients’ files.
Clinical Measurements
Presence of peri-implant bone loss was determined by radiographic evaluation. The following clinical measurements were performed immediately before treatment, as well as at 1.5 and 3 months after treatment using a plastic probe (Williams, Hu-friedy Mfg Co Inc., Chicago, IL, USA) with light pressure( 0.2-0.3 N):
Gingival index (GI); assessed according to Löe & Sillness GI 1963
BOP; evaluated according to papillary bleeding index (PBI); Saxer & Mühlemann 1975
PPD; measured from the mucosal margin to the bottom of the probeable pocket
Mucosal recession (MR); measured as the distance from the mucosal margin to the margin of prosthesis
Clinical attachment loss (CAL); measured from the prosthesis margin to the bottom of the probeable pocket
PPD and CAL were evaluated at six aspect per implant (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual). MR was measured at two sites per implant (buccal, lingual). All measurements were made by one experienced blind investigator.
Treatment Protocols
All implants that met the inclusion criteria underwent mechanical debridement using plastic curettes (Implacare– IMPHDL6, Hu-friedy Mfg Co Inc., Chicago, IL, USA), followed by pocket irrigation with sterile saline. Hand instrumentation was carried out until the operator was assured that the implant surfaces were adequately debrided and no time restriction was considered.
After completion of closed surface scaling, implants of the same disease, in each patient, were randomly allocated as PDT group and control group.
In the control group, no further treatment was performed. Whereas in the PDT group, closed surface scaling was followed by single-episode of PDT. A high medium photosensitizer (Fotösan, CMS Dental, Denmark) was injected inside the instrumented peri-implant pocket with a thin blunt needle, starting from the apical portion of the pocket. The photosensitizer was left in the pocket for 3 minutes. Subsequently, the light emitting device (LED, Fotösan, CMS Dental, Denmark) with the wavelength of 630 nm and the intensity of 2000 mW/cm2, with a special tip was placed at the depth of pocket, according to the manufacturer’s instruction. The device used in this research for implant surface irradiation, was in contact with 6 aspects per implant. All aspects of implants were irradiated for 20 seconds, making a total of 2 minutes. Chemical composition of the photosensitizer used is presented in Table 2.
Table 2 . Chemical Composition of High Medium FotoSan Agent.
| Ingredient | W/W % |
| Demineralized water | 74.71 |
| Glycerol | 23.77 |
| Xanthan gum ( keltrol) | 1.51 |
| Toluidine blue | 0.01 |
Oral hygiene instruction individualized for every subject, according to the type of prosthesis, was given at the first appointment and was confirmed in every recalls. No chemical and antimicrobial agent was instructed. All treatment procedures were performed by the same experienced operator. Clinical parameters were reevaluated at 1.5 and 3 month after treatment.
Statistical Analysis
Implants were chosen as unites of analysis. Descriptive statistical analysis was performed using frequency and percentage for the qualitative variables, while mean and standard deviation were computed for the quantitative variables. Statisticians were not aware of groups. Normal distribution was looked for by the Kolmogorov–Smirnov test. Intra-group differences were assessed by Wilcoxon signed-rank test. Comparison between the two groups was performed by Mann-Whitney test/ parametric or nonparametric as appropriate. To evaluate the changes over time within the groups, Friedman analysis was used. SPSS package version 11.0, (IBM, Chicago, IL. USA) was used for all calculations. Results were considered significant when P <0.05.
Results
All subjects completed the 3-month evaluation period. No adverse effects such as discomfort, pain or infections were reported by any of the subjects, in both groups. None of the smokers changed their habit during the 3-month trial. At the baseline examination, there was no statistical significant difference in any of the investigated parameters, between control and PDT group. The mean PD, MR and CAL values at baseline and after 3 and 6 months as assessed in both groups are presented in Table 3.
Table 3 . Clinical Parameters (mm, Mean ± SD) at Baseline, 1.5 and 3 Months After Therapy in Case and Control Groups .
| Parameters | Baseline | After 1.5 Months | The Difference Baseline to 1.5 Months | After 3 Months | The Difference 1.5 to 3 Months | The Dfference Baseline to 3 Months |
| PPD | ||||||
| Case | 5.36 ± 1.13 | 3.75 ± 0.9 | 1.6 ± 0.61a | 3.13 ± 0.54 | 1.6 ± 0.61a | 2.2 ± 0.84a |
| Control | 5.08 ± 1.47 | 5.09 ± 1.5 | 0.01 ± 0.9 | 5.08 ± 1.5 | 0.01 ± 0.9 | 0 |
| CALb | ||||||
| Casec | 7.36 ± 1.57 | 5.57 ± 1.09 | 1.79 ± 0.96a | 4.79 ± 1.36 | 0.78 ± 0.6a | 2.57 ± 0.88d |
| Control | 7.16 ± 1.4 | 7.17 ± 1.4 | 0.01 ± 0.07 | 7.18 ± 1.4 | 0.01 ± 0.07 | 0.02 ± 0.07 |
| MR | ||||||
| Case | 2 ± 1.76 | 1.9 ± 1.59 | 0.1 ± 0.32 | 1.9 ± 1.63 | 0 | 0.1 ± 0.32 |
| Control | 2.2 ± 1.68 | 2.2 ± 1.68 | 0 | 2.2 ± 1.68 | 0 | 0 |
aStatistically significant differences.
bSignificant differences between groups were analyzed using Mann-Whitney test.
cSignificant differences intra-groups were analyzed using Wilcoxon signed-rank test.
dSignificant differences during three-time point were analyzed using Friedman test.
Probing Pocket Depth
At 1.5 and 3 months after treatment, there was no statistically significant difference in the control group, with regard to PPD (P>0.5). A slight but non-significant increase of mean PPD was observed 1.5 month after mechanical debridement. The mean PPD of 5.36±1.13 mm at baseline decreased to 3.13±0.54 mm at 3 months after therapy, in the PDT group, and the Wilcoxon signed-rank test revealed a statistically significant difference (P<0.001). Regarding PPD, significant differences were observed inter-groups 1.5 and 3 months after therapy (P<0.001).
Clinical Attachment Loss
CAL reductions between baseline and 1.5 and 3 months in the PDT group were 1.79 mm and 2.57 mm respectively (P<0.001), whereas the CAL reductions in the control group were 0.01 mm and 0.02 mm (P>0.5 ). The majority of PDT implants revealed a CAL gain of 2 mm. The mean PD reduction and CAL gain tended to be higher in 1.5-month recall than 3-month recall in implants treated with PDT.
Mucosal Recession
Neither the test nor the control group showed differences between baseline, 1.5 and 3 months, regarding MR. Statistical analysis demonstrated that there was no significant difference in the groups, at any time point (P>0.5).
Bleeding on Probing
The number of implants with BOP and the degree of bleeding was reduced 1.5 months after PDT, compared to the baseline (P<0.05). Three months after therapy, in the test group, bleeding score was significantly decreased, while the degree of bleeding was slightly higher at 3 months after closed surface scaling; and intra-group difference was not statistically significant (P>0.5).
Mean BOP values were significantly reduced in the test group after 1.5 and 3 months (P<0.001, P<0.0001 respectively).
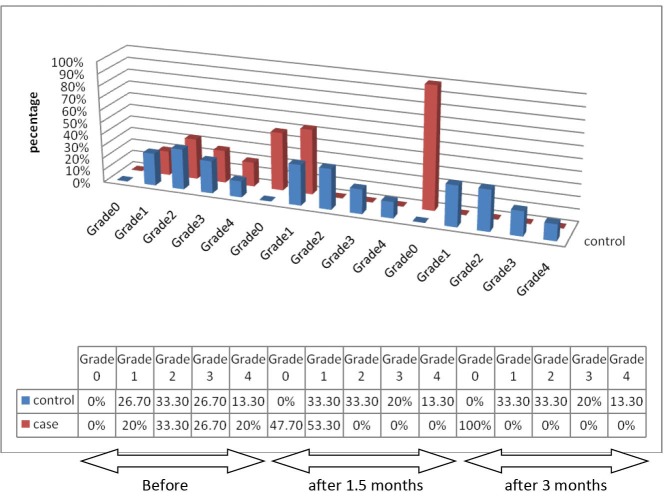
After 3 months, all implants in PDT group had stopped bleeding. BOP changes in test and control groups over the time are presented in Figure 1.
Figure 1 .
Frequency Distribution of Subjects in Relation to BOP in Test and Control Groups Over the Time.
Gingival Index
PDT resulted in significant improvement of GI in the test group, in comparison with baseline and control groups (P<0.01). There was no change in control group (P>0.5). GI changes in test and control groups over the time are presented in Figure 2.
Figure 2 .
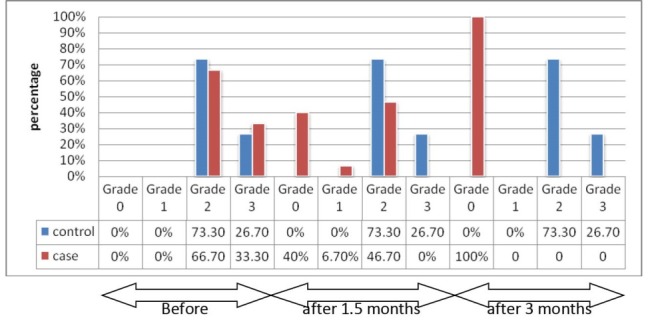
Frequency Distribution of Subjects in Relation to GI in Test and Control Groups Over the Time.
Discussion
The present randomized controlled clinical trial was designed to assess the effectiveness of antimicrobial PDT on peri-implant diseases. The study revealed that the adjunctive use of antimicrobial PDT following mechanical debridement resulted in statistically significant GI, BOP, PPD reductions as well as CAL gains.
The reason for improvement observed in the experimental group could be explained in this way:
Peri-implant diseases have infectious nature and the disease manifestation is due to inflammatory response to the accumulation of bacterial biofilm.26,27 In other words the clinical signs of peri-implant diseases (increasing BOP, CAL, and PPD) point to the tissue inflammation (apical migration of junctional epithelium, increasing blood vessels and immune cell infiltration) caused by plaque formation.28 Considering the etiology of these diseases, the main goal for treatment is to decrease bacterial load. Previous in vivo and in vitro studies have reported efficient elimination of pathogens causing peri-implant diseases, using the photodynamic method.29-32 Thus, it is reasonable that elimination of pathogenic bacteria and subsequently suppression of immune responses lead to disease halt and improvement of clinical parameters (BOP, CAL, PPD, and GI).
However, various bacterial species may have different sensitivity to this method, according to cell morphology, but it is likely that the reduction in total number of bacteria can reestablish the equilibrium between host defense and pathogens, which will arrest the progression of diseases. And the complete elimination of bacteria is not necessary.33
In the present study, mechanical debridement using plastic curettes designed for implant surface decontamination failed to improve clinical indices around implants studied, in the control group. These findings are consistent with other studies.34 The previous microbiological and clinical studies, demonstrated that mechanical debridement alone was not efficient for decontamination of implant surfaces and did not give rise to improvement of the clinical situation.35 It is very difficult to eliminate bacteria from implant surfaces using mechanical methods alone. Several factors are associated with the limited clinical outcomes. Available instruments are not proper for implant decontamination and cannot reach infected surfaces and tissues.36 Implant surface microstructures, designed to enhance osseointegration, protect the bacteria.37 Furthermore, high affinity of Staphylococcus aureus to titanium surfaces is reported in related studies. In vitro studies have shown that scraping an implant surface fails to remove S. aureus from implant surfaces.38
However, it should be emphasized that mechanical disturbance of microbial plaque and calculus removal is the basic step for treatment of any plaque induced disease such as peri-implant diseases. Apparently, using low-level laser will not remove submucosal plaque and calculus in the peri-implant pocket. In brief, mechanical disturbance and removal of supra and submucosal bacterial deposits are essential in the treatment of peri-implant infections.
Researchers usually evaluate the efficacy of treatment by assessing clinical parameters, as we used in this investigation. Therefore a question raises, that whether clinical indices used for periodontal examination are reliable for assessing peri-implant tissues condition or not. In order to reply to this question, several factors should be considered. In spite of structural differences in supporting tissues between teeth and implants, previous histological studies demonstrated that probing using a light and controlled force (0.2–0.3 N) as we used in the present study, is a reliable and valuable diagnostic tool.39
In addition, besides sufficient cleaning, good oral hygiene by patients themselves is so important to minimize plaque reformation and achieve the best therapeutic results. So, at each time point all subjects received oral hygiene instructions on an individual basis. Subjects were asked not to use any mouthrinse and chemical or antimicrobial products during the study. It seemed that the clinical outcomes in the test group would not be maintained at 3-month follow-up, unless biofilm formation on the implant surfaces was interfered. For the treatment of peri-implant diseases, oral hygiene instructions should be undertaken in order to enhance oral hygiene of natural teeth and dental implants.40
Unfortunately, studies on treatment options for peri-implant diseases are generally limited in number, with small sample sizes and short follow up periods.41 However, clinical data must be interpreted from controlled and randomized studies, with big sample size. Despite the small sample size in this investigation, it should also be mentioned that strict inclusion criteria followed in the present study, made our results valid. Because of the small number of subjects, we could not analyze demographics (e.g. smoking) as risk factors for peri-implant diseases.
Taken together, further comprehensive studies with higher number of cases should be performed in order to evaluate the efficacy of PDT in the treatment of peri-implant diseases. PDT is considered to resolve the limitation of conventional methods and can be successfully used in the treatment of peri-implant diseases. Further studies are needed to confirm our results.
Conclusion
Within the limits of the present study, it can be concluded that antimicrobial PDT following closed surface scaling resulted in an improvement of clinical parameters, in the treatment of peri-implant diseases.
Ethical Considerations
This study have been approved by ethical committee of Islamic Azad University of medical science
Conflict of Interests
The authors declare that they have no conflict of interest related to this study. The study was self-funded by the authors.
Acknowledgments
The authors wish to thank Mr. N. Valai and Miss M. Fakhrzadegan, statisticians in Islamic Azad University, Tehran, Iran, for carrying out all required statistics.
Please cite this article as follows: Karimi MR, Hasani A, Khosroshahian S. Efficacy of antimicrobial photodynamic therapy as an adjunctive to mechanical debridement in the treatment of peri-implant diseases: a randomized controlled clinical trial. J Lasers Med Sci. 2016;7(3):139-145. doi:10.15171/jlms.2016.24.
References
- 1.Leonhardt A, Gröndahl K, Bergström G, Lekholm U. Long-term follow-up of osseointegrated titanium implants using clinical, radiographic and microbiological parameters. Clin Oral Implants Res. 2002;13(2):127–132. doi: 10.1034/j.1600-0501.2002.130202.x. [DOI] [PubMed] [Google Scholar]
- 2.Takasaki A, Aoki A, Mizotani K, Schwarz F, Sculean A, Wang CY. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000. 2009;51:1–32. doi: 10.1111/j.1600-0757.2009.00302.x. [DOI] [PubMed] [Google Scholar]
- 3.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontal. 2008;35(Suppl 8):286–291. doi: 10.1111/j.1600-051x.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 4.Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011;38(9):872–878. doi: 10.1111/j.1600-051x.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 5.Shibli J, Melo L, Ferrari D, Figueiredo L, Faveri M, Feres M. Comparison of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin Oral Implants Res. 2008;19(10):975–982. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- 6.Zitzmann NU, Abrahamsson I, Berglundh T, Lindhe J. Soft tissue reactions to plaque formation at implant abutments with different surface topography An experimental study in dogs. J Clin Periodontol. 2002;29(5):456–461. doi: 10.1034/j.1600-051x.2002.290511.x. [DOI] [PubMed] [Google Scholar]
- 7.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–189. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 8.Parlor A, Bosshardt D, Cetiner D. et al. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin Oral Implants Res. 2009;20(4):391–399. doi: 10.1111/j.1600-0501.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- 9.Karring E, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri-implantitis by the Vector system. Clin Oral Implants Res. 2005;16(3):288–293. doi: 10.1111/j.1600-0501.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 10.Porras R, Anderson G, Caffesse R, Narendran S, Trejo P. Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol. 2002;73(10):1118–1125. doi: 10.1902/jop.2002.73.10.1118. [DOI] [PubMed] [Google Scholar]
- 11.Person L, Mouhji J, Berglundh T, Sennerby L, Lindhe J. Carbon dioxide laser and hydrogen peroxide conditioning in the treatment of periimplantitis: an experimental study in the dog. Clin Implant Dent Relat Res. 2004;6(4):230–238. doi: 10.1111/j.1708-8208.2004.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 12.Maximo M, de Mendonca A, Renata santos V, Figueiredo L, Feres M, Duarte P. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009;20(1):99–108. doi: 10.1111/j.1600-0501.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 13.Nociti F, Caffesse R, Sallum E, Machado M, Stefani C, Sallum A. Evaluation of guided bone regeneration and/or bone grafts in the treatment of ligature-induced peri-implantitis defects: a morphometric study in dogs. J Oral Implantol. 2000;26(4):244–249. doi: 10.1563/1548-1336(2000)026<0244:EOGBRA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Fürst M, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res. 2007;18(4):501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 15.Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009;80(11):1824–1832. doi: 10.1902/jop.2009.090273. [DOI] [PubMed] [Google Scholar]
- 16.Kreisler M, Kohnen W, Christoffers AB. et al. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er:YAG laser and an air powder system. Clin Oral Implants Res. 2005;16(1):36–43. doi: 10.1111/j.1600-0501.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 17.Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009;80(11):1824–32. doi: 10.1902/jop.2009.090273. [DOI] [PubMed] [Google Scholar]
- 18.Sigusch BW, Pfitzner A, Albrecht V, Glockmann E. Efficacy of phodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76(7):1100–1115. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 19.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86(8):694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 20.Pfitzner A, Sigusch BW, Albrecht V, Glockmann E. Killing of periodontopathogenic bacteria by photodynamic therapy. J Periodontol. 2004;75(10):1343–1349. doi: 10.1902/jop.2004.75.10.1343. [DOI] [PubMed] [Google Scholar]
- 21.Chan Y, Lai CH. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci. 2003;18(1):51–55. doi: 10.1902/jop.2004.75.10.1343. [DOI] [PubMed] [Google Scholar]
- 22.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol. 1998;68(3):370–376. doi: 10.1111/j.1751-1097.1998.tb09694.x. [DOI] [PubMed] [Google Scholar]
- 23.Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: State of the art. J Photochem Photobiol B. 2005;79(2):159–70. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Haas R, Baron M, Dortbudak O, Watzek G. Lethal photosensitization, autogenous bone, and e-PTFE membrane for the treatment of peri-implantitis: preliminary results. Int J Oral Maxillofac Implants. 2000;15(3):374–382. [PubMed] [Google Scholar]
- 25.Sarkar S, Wilson M. Lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. J Periodontal Res. 1993;28(3):204–210. doi: 10.1111/j.1600-0765.1993.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 26.Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth An experimental study in the dog. Clin Oral Implants Res. 1992;3(1):1–8. doi: 10.1034/j.1600-0501.1992.030101.x. [DOI] [PubMed] [Google Scholar]
- 27.Berglundh T, Gislason O, Lekholm U, Sennerby L, Lindeh J. Histopathological observation on human peri implantitis lesions. J Clin Periodontol. 2004;31(5):341–347. doi: 10.1111/j.1600-051X.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 28.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86(8):694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 29.Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implantants Res. 2001;12(2):104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayek RR, Araujo NS, Gioso MA. et al. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature- induced peri-implantitis in dogs. J Periodontol. 2005;76(8):1275–1281. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 31.Shibli JA, Martins M, Nociti F, Garcia V, Marcantonio E. Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary study in dogs. J Periodontol. 2003;74(3):338–345. doi: 10.1902/jop.2003.74.3.338. [DOI] [PubMed] [Google Scholar]
- 32.Haas R, Dörtbudak O, Mensdorff‐pouilly N, Mailath G. Elimination of bacteria on different implant surfaces through photosensitization and soft laser An in vitro study. Clin Oral Implants Res. 2002;8(4):249–254. doi: 10.1034/j.1600-0501.1997.080401.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol. 2011;38(3):276–284. doi: 10.1111/j.1600-051X.2010.01690.x. [DOI] [PubMed] [Google Scholar]
- 34.Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study I: Clinical results. J Clin Periodontol. 2009;36(7):604–609. doi: 10.1111/j.1600-051X.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 35.Renvert S, Lessem J, Dahlén G, Renvert H, Lindhal C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2008;79(5):836–844. doi: 10.1902/jop.2008.070347. [DOI] [PubMed] [Google Scholar]
- 36.Salvi G, Persson G, Heitz-Mayfield L, Frei M, Lang N. Adjunctive local antibiotic therapy in the treatment of peri-implantitis ll: clinical and radiographic outcome. Clin Oral Implants Res. 2007;18(3):281–285. doi: 10.1111/j.1600-0501.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 37.Persson GR, Samuelsson E, Lindahl C, Renvert S. Mechanical non-surgical treatment of peri-implantitis: a single-blinded randomized longitudinal clinical study II Microbiological results. J Clin Periodontol. 2010;37(6):563–573. doi: 10.1111/j.1600-051x.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 38.Salvi GE, Fürt MM, Lang NP, Persson GR. One-year bacterial colonization patterns of staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res. 2008;19(3):242–248. doi: 10.1111/j.1600-0501.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 39.Gerber JA, Tan WC, Balmer TE, Salvi GE, Lang NP. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin Oral Implants Res. 2009;20(1):75–78. doi: 10.1111/j.1600-0501.2008.01601.x. [DOI] [PubMed] [Google Scholar]
- 40.Gosau M, Habnel M, Schwarz F, Gerlach T, Reichert T, Bürgers R. Effect of six different peri-implantitis disinfection methods on invivo human oral biofilm. Clin Oral Implants Res. 2010;21(8):866–872. doi: 10.1111/j.1600-0501.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- 41.Kotsoovilis S, Karoussis I, Trianti M, Fourmousis I. Therapy of peri-implantitis: a systemic review. J Clin Periodontol. 2008;35(7):621–629. doi: 10.1111/j.1600-051x.2008.01240.x. [DOI] [PubMed] [Google Scholar]