Abstract
Background and Purpose
To prevent strokes that may occur as the first manifestation of atrial fibrillation (AF), screening programs have been proposed to identify patients with undiagnosed AF who may be eligible for treatment with anticoagulation. However, the frequency with which patients with AF present with stroke as the initial manifestation of the arrhythmia is unknown.
Methods
We estimated the frequency with which AF may present as a stroke in 1,809 community-based Framingham Heart Study participants with first-detected AF and without prior strokes, by tabulating frequency of strokes occurring on the same day, within 30 days prior, 90 days prior, and 365 days prior to first-detected AF. Using previously reported AF incidence rates, we estimated the incidence of strokes that may represent the initial manifestation of AF.
Results
We observed 87 strokes that occurred up to one year prior to AF detection, corresponding to 1.7% on the same day, 3.4% within 30 days prior, 3.7% within 90 days prior, and 4.8% up to one year prior to AF detection. We estimated that strokes may present as the initial manifestation of AF at a rate of 2 to 5 per 10,000 person-years, in both men and women.
Conclusion
We observed that stroke is an uncommon but measureable presenting feature of AF. Our data imply that emphasizing cost-effectiveness of population-wide AF screening efforts will be important given the relative infrequency with which stroke represents the initial manifestation of AF.
Keywords: stroke, atrial fibrillation, atrial flutter
Since atrial fibrillation (AF) may be paroxysmal and asymptomatic, stroke can be the first clinically recognized manifestation of AF. Recently, large-scale screening efforts for AF have been designed with the goal of preventing strokes through the early identification of AF.1–3 Opportunistic screening for AF in patients over age 65 has been endorsed.4 The cost-effectiveness of such efforts may be significantly influenced by the frequency with which individuals with AF initially present with a stroke. Yet, to date, cost effectiveness data2, 3, 5, 6 have generally relied on assumptions of stroke rates observed in patients with established AF, whereas incidence rates of undiagnosed AF presenting as a stroke remain unclear. To characterize strokes occurring as the initial manifestation of AF, we assessed incident strokes that occurred up to one year prior to AF detection in the community-based Framingham Heart Study (FHS).
METHODS
Participants from the FHS Original and Offspring cohorts were eligible for this study.7, 8 Of 2,032 individuals with AF, we excluded those who did not attend a Study examination within 10 years prior to first-detected AF (n=223). We included 1,809 individuals with incident AF between 1951 and 2013. The Boston University Medical Center Institutional Review Board approved study protocols, and participants provided consent at each cycle.
Participants were classified as having AF if atrial fibrillation or flutter was present on an electrocardiogram obtained at FHS clinic visit (about every two years for Original cohort and every four to eight years for Offspring cohort) or was identified in an encounter with external clinicians, by ambulatory cardiac monitoring, or documented in hospital records, as previously described.9 Strokes were defined as acute-onset neurological deficits of presumed or definite vascular etiology persisting for at least 24 hours, whereas transient ischemic attacks were defined as those lasting less than 24 hours. Imaging studies, noninvasive vascular studies, cardiac evaluations, and information from autopsies were used to ascertain stroke status. Two cardiologists adjudicated first-detected AF events and two neurologists adjudicated all strokes and transient ischemic attacks.
To determine the proportion of individuals with AF who presented with stroke prior to AF detection, we tabulated the number of individuals with strokes occurring at or prior to AF in several temporal windows (i.e., same day, within 30 days prior, within 90 days prior, and within 365 days prior). We selected 365 days a priori, under the assumption that strokes were less likely to be related to AF the longer the window between stroke and first detected AF. We stratified stroke events by sex and age of first-detected AF (<65, 65–74, and ≥75 years). To estimate the incidence of stroke occurring as the initial manifestation of AF, we multiplied the proportion of individuals with first-detected AF with a stroke by previously published FHS AF incidence rates.10 Analyses were conducted using SAS version 9.3 for Windows (SAS Institute, Cary, NC).
RESULTS
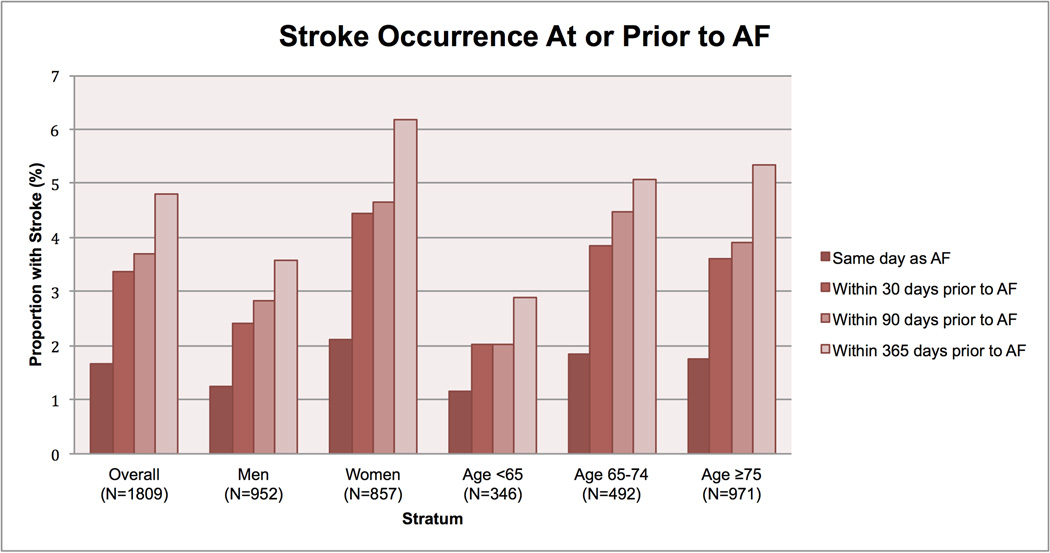
Of the 1,809 individuals with incident AF, the mean age at first-detected AF was 74.6±11.2 years and 857 (47%) were women. Eighty-seven strokes occurred up to one year prior to AF detection, 82 (94%) of which were ischemic. Thirty (1.7%) individuals had strokes that occurred on the same day as the first-detected AF event, 61 (3.4%) occurred within 30 days prior to AF detection, 67 (3.7%) within 90 days, and 87 (4.8%) up to one year prior (Figure). As expected, the proportion of individuals with stroke was generally greater among women than men, and was greater with older age. The results did not differ substantively if the data were restricted to ischemic strokes.
Figure. Stroke occurrence at or prior to atrial fibrillation detection.
The proportion of individuals with atrial fibrillation in whom a stroke occurred at or prior to first-detected atrial fibrillation is displayed stratified by sex and by age.
By extrapolating these observations (Table), we estimate that for both men and women, approximately 2 to 5 individuals per 10,000 person-years may present with stroke as the initial manifestation of AF (based on an AF incidence rate of 134 cases per 10,000 person-years in men, and 86 cases per 10,000 person-years in women).10
Table.
Range of estimated incidence rates for strokes caused by atrial fibrillation.
| Incidence of AF (per 10,000 person-years)* |
Proportion with strokes occurring on the same day as AF detection (%) |
Proportion with strokes occurring within 365 days prior to AF detection (%) |
Range of incidence estimates for stroke events caused by AF (per 10,000 person-years) |
|
|---|---|---|---|---|
| Men | 134 | 1.3 | 3.6 | 1.7–4.8 |
| Women | 86 | 2.1 | 6.2 | 1.8–5.3 |
DISCUSSION
In our community-based sample of 1,809 individuals with AF detected over a 60-year period, we observed that between 2% to 5% presented with a stroke or transient ischemic attack at or in near proximity prior to the AF diagnosis. Our data extend prior knowledge by suggesting that stroke is an uncommon but measurable presenting feature of AF.
Assuming that strokes occurring in close proximity to an AF diagnosis are attributable to AF and are preventable with anticoagulation, our observations imply that population-wide AF screening efforts with pulse checks, mobile technology, or other modalities11 may be effective for reducing the incidence of stroke. However, cost-effective screening efforts are paramount, given the relative infrequency with which strokes present as the first manifestation of AF. Cost-effectiveness analyses will be informed by future data addressing the prevalence of undiagnosed AF in different stroke risk strata, prevalence of paroxysmal versus persistent AF (the latter of which may be easier to identify with AF screening interventions), as well as the accuracy, modality, patient adherence, and costs associated with different screening efforts. Such efforts are likely to be of greatest value among individuals with the highest stroke risks, who are eligible for anticoagulation if AF is identified through screening.
We acknowledge that we may have underestimated the frequency of AF-associated stroke, particularly if such strokes were severely disabling or fatal, if AF was unrecognized, or if AF occurred only as an isolated episode. Moreover, though we examined time windows up to 365 days preceding an AF event, we acknowledge that strokes occurring more than 365 days prior to an AF event may be related to undetected arrhythmia. Our sample was predominantly of European ancestry, and therefore our findings may not be generalizable to other racial or ethnic groups. Our analysis spanned multiple decades and era-specific factors may have influenced the probability of AF and stroke detection. Data from the Framingham Heart Study indicate that recently observed growth in AF incidence was partially attributable to increases in cardiac rhythm surveillance over time.10 Not all strokes may have been of cardioembolic origin from AF. Some of the AF events may have occurred as a consequence of stroke,12 rather than being causally related to the stroke. However, most AF observed at the time of an acute stroke has been reported to coexist long-term.13
CONCLUSIONS
In conclusion, we observed that strokes are uncommon but may represent the first presentation of AF. The utility and cost-effectiveness of efforts to reduce stroke by identifying individuals with undiagnosed AF require prospective evaluation.
Acknowledgments
None
SOURCES OF FUNDING
NIH grants K23HL114724 (Lubitz), 2R01HL092577 and 1R01HL128914 (Ellinor and Benjamin), 1R01HL102214 (Benjamin), R01HL104156 and K24HL105780 (Ellinor), R01NS017950 (Seshadri), HHSN268201500001I and N01-HC-25195 (FHS); Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 (Lubitz); American Heart Association Established Investigator Award 13EIA14220013 (Ellinor).
DISCLOSURES
Dr. Lubitz has received consulting support from St. Jude Medical. Dr. Ellinor receives grant funding from Bayer HealthCare to the Broad Institute to study the mechanisms of AF, and consulting support from Quest Diagnostics. Dr. Walkey has received funding from UptoDate. Dr. Benjamin receives research support from the NIH/NHLBI.
REFERENCES
- 1.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 2.Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9:iii–iv. ix–x, 1–74. doi: 10.3310/hta9400. [DOI] [PubMed] [Google Scholar]
- 3.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014;111:1167–1176. doi: 10.1160/TH14-03-0231. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO) European heart journal. 2016 [Google Scholar]
- 5.Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023–1029. doi: 10.1093/europace/euv083. [DOI] [PubMed] [Google Scholar]
- 6.Maeda K, Shimbo T, Fukui T. Cost-effectiveness of a community-based screening programme for chronic atrial fibrillation in Japan. J Med Screen. 2004;11:97–102. doi: 10.1258/096914104774061092. [DOI] [PubMed] [Google Scholar]
- 7.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healey JS, Sandhu RK. Are We Ready for Mass Screening to Detect Atrial Fibrillation? Circulation. 2015;131:2167–2168. doi: 10.1161/CIRCULATIONAHA.115.017288. [DOI] [PubMed] [Google Scholar]
- 12.Vingerhoets F, Bogousslavsky J, Regli F, Van Melle G. Atrial fibrillation after acute stroke. Stroke. 1993;24:26–30. doi: 10.1161/01.str.24.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Lin HJ, Wolf PA, Benjamin EJ, Belanger AJ, D'Agostino RB. Newly diagnosed atrial fibrillation and acute stroke. The Framingham Study. Stroke; a journal of cerebral circulation. 1995;26:1527–1530. doi: 10.1161/01.str.26.9.1527. [DOI] [PubMed] [Google Scholar]